A Label and Probe-Free Zika Virus Immunosensor Prussian Blue@carbon Nanotube-Based for Amperometric Detection of the NS2B Protein
- PMID: 34065688
- PMCID: PMC8156682
- DOI: 10.3390/bios11050157
A Label and Probe-Free Zika Virus Immunosensor Prussian Blue@carbon Nanotube-Based for Amperometric Detection of the NS2B Protein
Abstract
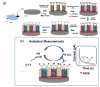
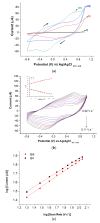
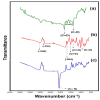
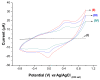
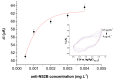
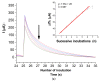
Zika virus (ZIKV) is a mosquito-borne infection, predominant in tropical and subtropical regions causing international concern due to the ZIKV disease having been associated with congenital disabilities, especially microcephaly and other congenital abnormalities in the fetus and newborns. Development of strategies that minimize the devastating impact by monitoring and preventing ZIKV transmission through sexual intercourse, especially in pregnant women, since no vaccine is yet available for the prevention or treatment, is critically important. ZIKV infection is generally asymptomatic and cross-reactivity with dengue virus (DENV) is a global concern. An innovative screen-printed electrode (SPE) was developed for amperometric detection of the non-structural protein (NS2B) of ZIKV by exploring the intrinsic redox catalytic activity of Prussian blue (PB), incorporated into a carbon nanotube-polypyrrole composite. Thus, this immunosensor has the advantage of electrochemical detection without adding any redox-probe solution (probe-less detection), allowing a point-of-care diagnosis. It was responsive to serum samples of only ZIKV positive patients and non-responsive to negative ZIKV patients, even if the sample was DENV positive, indicating a possible differential diagnosis between them by NS2B. All samples used here were confirmed by CDC protocols, and immunosensor responses were also checked in the supernatant of C6/36 and in Vero cell cultures infected with ZIKV.
Keywords: NS2B; Prussian blue; Zika virus; point-of-care testing; probeless; reagentless.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A Redox-Probe-Free Immunosensor Based on Electrocatalytic Prussian Blue Nanostructured Film One-Step-Prepared for Zika Virus Diagnosis.Biosensors (Basel). 2022 Aug 10;12(8):623. doi: 10.3390/bios12080623. Biosensors (Basel). 2022. PMID: 36005020 Free PMC article.
-
NS1 glycoprotein detection in serum and urine as an electrochemical screening immunosensor for dengue and Zika virus.Anal Bioanal Chem. 2021 Aug;413(19):4873-4885. doi: 10.1007/s00216-021-03449-7. Epub 2021 Jun 21. Anal Bioanal Chem. 2021. PMID: 34152457
-
A serological point-of-care test for Zika virus detection and infection surveillance using an enzyme-free vial immunosensor with a smartphone.Biosens Bioelectron. 2020 Mar 1;151:111960. doi: 10.1016/j.bios.2019.111960. Epub 2019 Dec 24. Biosens Bioelectron. 2020. PMID: 31999574
-
Zika virus epidemic: an update.Expert Rev Anti Infect Ther. 2016 Dec;14(12):1127-1138. doi: 10.1080/14787210.2016.1245614. Epub 2016 Oct 21. Expert Rev Anti Infect Ther. 2016. PMID: 27712118 Review.
-
Zika virus structural biology and progress in vaccine development.Biotechnol Adv. 2018 Jan-Feb;36(1):47-53. doi: 10.1016/j.biotechadv.2017.09.004. Epub 2017 Sep 12. Biotechnol Adv. 2018. PMID: 28916391 Review.
Cited by
-
PEDOT-AuNPs-based impedimetric immunosensor for the detection of SARS-CoV-2 antibodies.Electrochim Acta. 2022 Feb 1;404:139757. doi: 10.1016/j.electacta.2021.139757. Epub 2021 Dec 18. Electrochim Acta. 2022. PMID: 34955549 Free PMC article.
-
A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor.Biosensors (Basel). 2022 Jul 21;12(7):545. doi: 10.3390/bios12070545. Biosensors (Basel). 2022. PMID: 35884348 Free PMC article.
-
Internet-of-medical-things integrated point-of-care biosensing devices for infectious diseases: Toward better preparedness for futuristic pandemics.Bioeng Transl Med. 2023 Jan 3;8(3):e10481. doi: 10.1002/btm2.10481. eCollection 2023 May. Bioeng Transl Med. 2023. PMID: 37206204 Free PMC article. Review.
-
A Methylene Blue-Enhanced Nanostructured Electrochemical Immunosensor for H-FABP Myocardial Injury Biomarker.Biosensors (Basel). 2023 Sep 7;13(9):873. doi: 10.3390/bios13090873. Biosensors (Basel). 2023. PMID: 37754107 Free PMC article.
-
Amino Acids, Peptides, and Proteins: Implications for Nanotechnological Applications in Biosensing and Drug/Gene Delivery.Nanomaterials (Basel). 2021 Nov 8;11(11):3002. doi: 10.3390/nano11113002. Nanomaterials (Basel). 2021. PMID: 34835766 Free PMC article. Review.
References
-
- Noorbakhsh F., Abdolmohammadi K., Fatahi Y., Dalili H., Rasoolinejad M., Rezaei F., Salehi-Vaziri M., Shafiei-Jandaghi N.Z., Gooshki E.S., Zaim M., et al. Zika virus infection, basic and clinical aspects: A review article. Iran. J. Public Health. 2019;48:20–31. doi: 10.18502/ijph.v48i1.779. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

