Cu-Catalyzed Arylation of Bromo-Difluoro-Acetamides by Aryl Boronic Acids, Aryl Trialkoxysilanes and Dimethyl-Aryl-Sulfonium Salts: New Entries to Aromatic Amides
- PMID: 34065691
- PMCID: PMC8156957
- DOI: 10.3390/molecules26102957
Cu-Catalyzed Arylation of Bromo-Difluoro-Acetamides by Aryl Boronic Acids, Aryl Trialkoxysilanes and Dimethyl-Aryl-Sulfonium Salts: New Entries to Aromatic Amides
Abstract
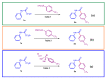
We describe a mechanism-guided discovery of a synthetic methodology that enables the preparation of aromatic amides from 2-bromo-2,2-difluoroacetamides utilizing a copper-catalyzed direct arylation. Readily available and structurally simple aryl precursors such as aryl boronic acids, aryl trialkoxysilanes and dimethyl-aryl-sulfonium salts were used as the source for the aryl substituents. The scope of the reactions was tested, and the reactions were insensitive to the electronic nature of the aryl groups, as both electron-rich and electron-deficient aryls were successfully introduced. A wide range of 2-bromo-2,2-difluoroacetamides as either aliphatic or aromatic secondary or tertiary amides were also reactive under the developed conditions. The described synthetic protocols displayed excellent efficiency and were successfully utilized for the expeditious preparation of diverse aromatic amides in good-to-excellent yields. The reactions were scaled up to gram quantities.
Keywords: C-C-coupling; amides; aryl trialkoxysilanes; boronic acids; catalysis; copper; dimethyl-aryl-sulfonium salts; fluorine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mechanochemical Defluorinative Arylation of Trifluoroacetamides: An Entry to Aromatic Amides.J Org Chem. 2023 Jan 20;88(2):863-870. doi: 10.1021/acs.joc.2c02197. Epub 2023 Jan 9. J Org Chem. 2023. PMID: 36622848 Free PMC article.
-
Copper/N,N-dimethylglycine catalyzed goldberg reactions between aryl bromides and amides, aryl iodides and secondary acyclic amides.Molecules. 2014 Aug 29;19(9):13448-60. doi: 10.3390/molecules190913448. Molecules. 2014. PMID: 25178065 Free PMC article.
-
Cerium Ammonium Nitrate (CAN)-Promoted C(sp2)-N Coupling of Secondary Amides with Aryl Boronic Acids: Entries to Tertiary Aryl Amides.Org Lett. 2025 Apr 11;27(14):3501-3505. doi: 10.1021/acs.orglett.5c00108. Epub 2025 Apr 2. Org Lett. 2025. PMID: 40173296
-
Sulfonium Salts as Acceptors in Electron Donor-Acceptor Complexes.Angew Chem Int Ed Engl. 2023 Jul 17;62(29):e202303104. doi: 10.1002/anie.202303104. Epub 2023 May 3. Angew Chem Int Ed Engl. 2023. PMID: 36959098 Free PMC article. Review.
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
Cited by
-
Mechanochemical Defluorinative Arylation of Trifluoroacetamides: An Entry to Aromatic Amides.J Org Chem. 2023 Jan 20;88(2):863-870. doi: 10.1021/acs.joc.2c02197. Epub 2023 Jan 9. J Org Chem. 2023. PMID: 36622848 Free PMC article.
-
Mechanochemical synthesis of aromatic ketones: pyrylium tetrafluoroborate mediated deaminative arylation of amides.Chem Sci. 2024 May 14;15(24):9155-9163. doi: 10.1039/d4sc00904e. eCollection 2024 Jun 19. Chem Sci. 2024. PMID: 38903233 Free PMC article.
References
-
- Harrington A., Tal-Gan Y. The Importance of Amide Protons in Peptide Drug Development. [(accessed on 8 November 2019)]; Available online: https://www.future-science.com/doi/abs/10.4155/fmc-2019-0238?journalCode.... - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

