Cu-Catalyzed Arylation of Bromo-Difluoro-Acetamides by Aryl Boronic Acids, Aryl Trialkoxysilanes and Dimethyl-Aryl-Sulfonium Salts: New Entries to Aromatic Amides
- PMID: 34065691
- PMCID: PMC8156957
- DOI: 10.3390/molecules26102957
Cu-Catalyzed Arylation of Bromo-Difluoro-Acetamides by Aryl Boronic Acids, Aryl Trialkoxysilanes and Dimethyl-Aryl-Sulfonium Salts: New Entries to Aromatic Amides
Abstract
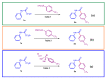
We describe a mechanism-guided discovery of a synthetic methodology that enables the preparation of aromatic amides from 2-bromo-2,2-difluoroacetamides utilizing a copper-catalyzed direct arylation. Readily available and structurally simple aryl precursors such as aryl boronic acids, aryl trialkoxysilanes and dimethyl-aryl-sulfonium salts were used as the source for the aryl substituents. The scope of the reactions was tested, and the reactions were insensitive to the electronic nature of the aryl groups, as both electron-rich and electron-deficient aryls were successfully introduced. A wide range of 2-bromo-2,2-difluoroacetamides as either aliphatic or aromatic secondary or tertiary amides were also reactive under the developed conditions. The described synthetic protocols displayed excellent efficiency and were successfully utilized for the expeditious preparation of diverse aromatic amides in good-to-excellent yields. The reactions were scaled up to gram quantities.
Keywords: C-C-coupling; amides; aryl trialkoxysilanes; boronic acids; catalysis; copper; dimethyl-aryl-sulfonium salts; fluorine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Harrington A., Tal-Gan Y. The Importance of Amide Protons in Peptide Drug Development. [(accessed on 8 November 2019)]; Available online: https://www.future-science.com/doi/abs/10.4155/fmc-2019-0238?journalCode.... - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

