Lincp21-RNA as Predictive Response Marker for Preoperative Chemoradiotherapy in Rectal Cancer
- PMID: 34065723
- PMCID: PMC8156811
- DOI: 10.3390/jpm11050420
Lincp21-RNA as Predictive Response Marker for Preoperative Chemoradiotherapy in Rectal Cancer
Abstract
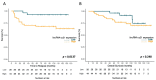
Preoperative chemoradiotherapy (CRT) is a standard treatment for locally advanced rectal cancer (RC) patients, but its use in non-responders can be associated with increased toxicities and resection delay. LincRNA-p21 is a long non-coding RNA involved in the p53 pathway and angiogenesis regulation. We aimed to study whether lincRNA-p21 expression levels can act as a predictive biomarker for neoadjuvant CRT response. We analyzed RNAs from pretreatment biopsies from 70 RC patients treated with preoperative CRT. Pathological response was classified according to the tumor regression grade (TRG) Dworak classification. LincRNA-p21 expression was determined by RTqPCR. The results showed that lincRNA-p21 was upregulated in stage III tumors (p = 0.007) and in tumors with the worst response regarding TRG (p = 0.027) and downstaging (p = 0.016). ROC curve analysis showed that lincRNA-p21 expression had the capacity to distinguish a complete response from others (AUC:0.696; p = 0.014). LincRNA-p21 was shown as an independent marker of preoperative CRT response (p = 0.047) and for time to relapse (TTR) (p = 0.048). In conclusion, lincRNA-p21 is a marker of advanced disease, worse response to neoadjuvant CRT, and shorter TTR in locally advanced RC patients. The study of lincRNA-p21 may be of value in the individualization of pre-operative CRT in RC.
Keywords: chemoradiotherapy; colorectal cancer; lincRNA-p21; long non-coding RNA; p53; predictive biomarker; rectal cancer.
Conflict of interest statement
All of the authors have reviewed and approved this version of the manuscript and agree with the decision to submit. None of the authors declare any conflicts of interest.
Figures


Similar articles
-
LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients.Life (Basel). 2020 Aug 31;10(9):172. doi: 10.3390/life10090172. Life (Basel). 2020. PMID: 32878005 Free PMC article.
-
p21 does, but p53 does not predict pathological response to preoperative chemoradiotherapy in locally advanced rectal cancer.J BUON. 2017 Nov-Dec;22(6):1463-1470. J BUON. 2017. PMID: 29332339
-
miR-21, miR-99b and miR-375 combination as predictive response signature for preoperative chemoradiotherapy in rectal cancer.PLoS One. 2018 Nov 2;13(11):e0206542. doi: 10.1371/journal.pone.0206542. eCollection 2018. PLoS One. 2018. PMID: 30388154 Free PMC article.
-
Diffusion-weighted MRI and MR- volumetry--in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer.Magn Reson Imaging. 2015 Feb;33(2):201-12. doi: 10.1016/j.mri.2014.08.041. Epub 2014 Nov 13. Magn Reson Imaging. 2015. PMID: 25460330
-
Do circulating long non-coding RNAs (lncRNAs) (LincRNA-p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cancer treated with chemoradiotherapy?Tumour Biol. 2016 Mar;37(3):3969-78. doi: 10.1007/s13277-015-4189-1. Epub 2015 Oct 19. Tumour Biol. 2016. PMID: 26482616
Cited by
-
LncRNAs Associated with Chemoradiotherapy Response and Prognosis in Locally Advanced Rectal Cancer.J Inflamm Res. 2021 Nov 27;14:6275-6292. doi: 10.2147/JIR.S334096. eCollection 2021. J Inflamm Res. 2021. PMID: 34866926 Free PMC article.
-
Non-coding RNA and reprogrammed mitochondrial metabolism in genitourinary cancer.Front Genet. 2024 Mar 13;15:1364389. doi: 10.3389/fgene.2024.1364389. eCollection 2024. Front Genet. 2024. PMID: 38544804 Free PMC article. Review.
-
Special Issue "Cancer Biomarker Research and Personalized Medicine".J Pers Med. 2022 Apr 5;12(4):585. doi: 10.3390/jpm12040585. J Pers Med. 2022. PMID: 35455701 Free PMC article.
References
-
- Bosset J.-F., Calais G., Mineur L., Maingon P., Stojanovic-Rundic S., Bensadoun R.-J., Bardet E., Beny A., Ollier J.-C., Bolla M., et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–190. doi: 10.1016/S1470-2045(13)70599-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

