Synthetic, Mesomorphic, and DFT Investigations of New Nematogenic Polar Naphthyl Benzoate Ester Derivatives
- PMID: 34065725
- PMCID: PMC8156059
- DOI: 10.3390/ma14102587
Synthetic, Mesomorphic, and DFT Investigations of New Nematogenic Polar Naphthyl Benzoate Ester Derivatives
Abstract
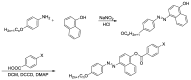
Four new non-symmetrical derivatives based on central naphthalene moiety, 4-((4-(alkoxy)phenyl) diazenyl)naphthalen-1-yl 4-substitutedbenzoate (In/x), were prepared, and their properties were investigated experimentally and theoretically. The synthesized materials bear two wing groups: an alkoxy chain of differing proportionate length (n = 6 and 16 carbons) and one terminal attached to a polar group, X. Their molecular structures were elucidated via elemental analyses and FT-IR and NMR spectroscopy. Differential scanning calorimetry (DSC) and polarized optical microscopy (POM) were carried out to evaluate their mesomorphic properties. The results of the experimental investigations revealed that all the synthesized analogues possess only an enantiotropic nematic (N) mesophase with a high thermal stability and broad range. Density functional theory (DFT) calculations were in accordance with the experimental investigations and revealed that all prepared materials are to be linear and planar. Moreover, the rigidity of the molecule increased when an extra fused ring was inserted into the center of the structural shape, so its thermal and geometrical parameters were affected. Energy gap predictions confirmed that the I16/c derivative is more reactive than other compounds.
Keywords: DFT; azo/ester; fused ring; liquid crystals materials; mesomorphic properties; optimized structures; thermal parameters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives.Molecules. 2021 Jul 28;26(15):4546. doi: 10.3390/molecules26154546. Molecules. 2021. PMID: 34361699 Free PMC article.
-
New wide-stability four-ring azo/ester/Schiff base liquid crystals: synthesis, mesomorphic, photophysical, and DFT approaches.RSC Adv. 2020 Mar 6;10(16):9643-9656. doi: 10.1039/c9ra10499b. eCollection 2020 Mar 2. RSC Adv. 2020. PMID: 35866044 Free PMC article.
-
Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies.Molecules. 2022 Jun 28;27(13):4150. doi: 10.3390/molecules27134150. Molecules. 2022. PMID: 35807398 Free PMC article.
-
Synthesis, Mesomorphic and Computational Characterizations of Nematogenic Schiff Base Derivatives in Pure and Mixed State.Molecules. 2021 Apr 2;26(7):2038. doi: 10.3390/molecules26072038. Molecules. 2021. PMID: 33918482 Free PMC article.
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
Cited by
-
Novel sulphonic acid liquid crystal derivatives: experimental, computational and optoelectrical characterizations.RSC Adv. 2021 Aug 17;11(45):27937-27949. doi: 10.1039/d1ra02517a. eCollection 2021 Aug 16. RSC Adv. 2021. PMID: 35480753 Free PMC article.
-
New Self-Organizing Optical Materials and Induced Polymorphic Phases of Their Mixtures Targeted for Energy Investigations.Polymers (Basel). 2022 Jan 23;14(3):456. doi: 10.3390/polym14030456. Polymers (Basel). 2022. PMID: 35160446 Free PMC article.
-
Synthesis, Phase Behavior and Computational Simulations of a Pyridyl-Based Liquid Crystal System.Molecules. 2021 Oct 24;26(21):6416. doi: 10.3390/molecules26216416. Molecules. 2021. PMID: 34770826 Free PMC article.
-
Synthesis, Optical Characterizations and Solar Energy Applications of New Schiff Base Materials.Materials (Basel). 2021 Jul 2;14(13):3718. doi: 10.3390/ma14133718. Materials (Basel). 2021. PMID: 34279287 Free PMC article.
-
Synthesis of New Liquid-Crystalline Compounds Based on Terminal Benzyloxy Group: Characterization, DFT and Mesomorphic Properties.Molecules. 2023 Apr 28;28(9):3804. doi: 10.3390/molecules28093804. Molecules. 2023. PMID: 37175214 Free PMC article.
References
-
- Luo Z., Peng F., Chen H., Hu M., Li J., An Z., Wu S.-T. Fast-response liquid crystals for high image quality wearable displays. Opt. Mater. Express. 2015;5:603–610. doi: 10.1364/OME.5.000603. - DOI
-
- Aleksandriiskii V., Novikov I., Kuvshinova S., Burmistrov V., Koifman O. Dielectric, optical and orientational properties of liquid crystalline 4-alkyloxy-4′-cyanoazoxybenzenes and 4-alkyloxy-4′-cyanoazobenzenes. J. Mol. Liq. 2016;223:1270–1276. doi: 10.1016/j.molliq.2016.09.064. - DOI
-
- Dave J.S., Bhatt H.S. Synthesis of liquid crystals with lateral methyl group and study of their mesomorphic properties. Mol. Cryst. Liq. Cryst. 2012;562:1–9. doi: 10.1080/15421406.2012.657129. - DOI
-
- Thaker B.T., Kanojiya J.B., Tandel R.S. Effects of Different Terminal Substituents on the Mesomorphic Behavior of Some Azo-Schiff Base and Azo-Ester-Based Liquid Crystals. Mol. Cryst. Liq. Cryst. 2010;528:120–137. doi: 10.1080/15421406.2010.504632. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

