Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D
- PMID: 34065735
- PMCID: PMC8155889
- DOI: 10.3390/ijms22105251
Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D
Abstract
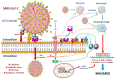
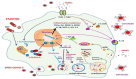
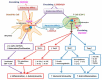
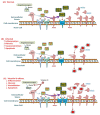
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is still an ongoing global health crisis. Immediately after the inhalation of SARS-CoV-2 viral particles, alveolar type II epithelial cells harbor and initiate local innate immunity. These particles can infect circulating macrophages, which then present the coronavirus antigens to T cells. Subsequently, the activation and differentiation of various types of T cells, as well as uncontrollable cytokine release (also known as cytokine storms), result in tissue destruction and amplification of the immune response. Vitamin D enhances the innate immunity required for combating COVID-19 by activating toll-like receptor 2. It also enhances antimicrobial peptide synthesis, such as through the promotion of the expression and secretion of cathelicidin and β-defensin; promotes autophagy through autophagosome formation; and increases the synthesis of lysosomal degradation enzymes within macrophages. Regarding adaptive immunity, vitamin D enhances CD4+ T cells, suppresses T helper 17 cells, and promotes the production of virus-specific antibodies by activating T cell-dependent B cells. Moreover, vitamin D attenuates the release of pro-inflammatory cytokines by CD4+ T cells through nuclear factor κB signaling, thereby inhibiting the development of a cytokine storm. SARS-CoV-2 enters cells after its spike proteins are bound to angiotensin-converting enzyme 2 (ACE2) receptors. Vitamin D increases the bioavailability and expression of ACE2, which may be responsible for trapping and inactivating the virus. Activation of the renin-angiotensin-aldosterone system (RAS) is responsible for tissue destruction, inflammation, and organ failure related to SARS-CoV-2. Vitamin D inhibits renin expression and serves as a negative RAS regulator. In conclusion, vitamin D defends the body against SARS-CoV-2 through a novel complex mechanism that operates through interactions between the activation of both innate and adaptive immunity, ACE2 expression, and inhibition of the RAS system. Multiple observation studies have shown that serum concentrations of 25 hydroxyvitamin D are inversely correlated with the incidence or severity of COVID-19. The evidence gathered thus far, generally meets Hill's causality criteria in a biological system, although experimental verification is not sufficient. We speculated that adequate vitamin D supplementation may be essential for mitigating the progression and severity of COVID-19. Future studies are warranted to determine the dosage and effectiveness of vitamin D supplementation among different populations of individuals with COVID-19.
Keywords: adaptive immunity; angiotensin-converting enzyme 2; coronavirus disease 2019; innate immunity; renin–angiotensin–aldosterone system; severe acute respiratory syndrome coronavirus 2; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

