Personalized Medicine Based on Nasal Epithelial Cells: Comparative Studies with Rectal Biopsies and Intestinal Organoids
- PMID: 34065744
- PMCID: PMC8156700
- DOI: 10.3390/jpm11050421
Personalized Medicine Based on Nasal Epithelial Cells: Comparative Studies with Rectal Biopsies and Intestinal Organoids
Abstract
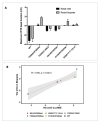
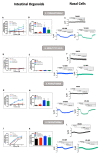
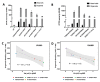
As highly effective CFTR modulator therapies (HEMT) emerge, there is an unmet need to find effective drugs for people with CF (PwCF) with ultra-rare mutations who are too few for classical clinical trials and for whom there are no drug discovery programs. Therefore, biomarkers reliably predicting the benefit from CFTR modulator therapies are essential to find effective drugs for PwCF through personalized approaches termed theranostics. Here, we assess CFTR basal function and the individual responses to CFTR modulators in primary human nasal epithelial (pHNE) cells from PwCF carrying rare mutations and compare these measurements with those in native rectal biopsies and intestinal organoids, respectively, in the same individual. The basal function in pHNEs shows good correlation with CFTR basal function in rectal biopsies. In parallel, CFTR rescue in pHNEs by CFTR modulators correlates to that in intestinal organoids. Altogether, results show that pHNEs are a bona fide theranostic model to assess CFTR rescue by CFTR modulator drugs, in particular for PwCF and rare mutations.
Keywords: CFTR; CFTR modulators; human nasal epithelial cells; intestinal organoids; rectal biopsies; theranostic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Elborn J.S., Davies J., Mall M.A., Flume P.A., Plant B. Current strategies for the long-term assessment, monitoring, and management of cystic fi brosis patients treated with CFTR modulator therapy. J. Cyst. Fibros. 2016;16:11–12. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

