Long-Term Preservation and Storage of Faecal Samples in Whatman® Cards for PCR Detection and Genotyping of Giardia duodenalis and Cryptosporidium hominis
- PMID: 34065892
- PMCID: PMC8151430
- DOI: 10.3390/ani11051369
Long-Term Preservation and Storage of Faecal Samples in Whatman® Cards for PCR Detection and Genotyping of Giardia duodenalis and Cryptosporidium hominis
Abstract
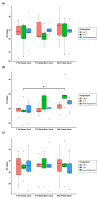
Preservation and conservation of biological specimens, including faecal samples, is a challenge in remote areas or poor-resource settings where the cold chain cannot be maintained. This study aims at evaluating the suitability of filter cards for long-term storage of faecal samples of animal and human origin positive to the diarrhoea-causing protozoan parasites, Giardia duodenalis and Cryptosporidium hominis. Three commercially available Whatman® Filter Cards were comparatively assessed: the FTA® Classic Card, the FTA® Elute Micro Card, and the 903 Protein Saver Card. Human faecal samples positive to G. duodenalis (n = 5) and C. hominis (n = 5) were used to impregnate the selected cards at given storage (1 month, 3 months, and 6 months) periods and temperature (-20 °C, 4 °C, and room temperature) conditions. Parasite DNA was detected by PCR-based methods. Sensitivity assays and quality control procedures to assess suitability for genotyping purposes were conducted. Overall, all three Whatman® cards were proven useful for the detection and molecular characterisation of G. duodenalis and C. hominis under the evaluated conditions. Whatman® cards represent a simple, safe, and cost-effective option for the transportation, preservation, and storage of faecal samples without the need of the cold chain.
Keywords: Cryptosporidium hominis; Giardia duodenalis; PCR; faeces; filter card; preservation; storage; transportation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Paulos S., Mateo M., de Lucio A., Hernández-de Mingo M., Bailo B., Saugar J.M., Cardona G.A., Fuentes I., Mateo M., Carmena D. Evaluation of five commercial methods for the extraction and purification of DNA from human faecal samples for downstream molecular detection of the enteric protozoan parasites Cryptosporidium spp., Giardia duodenalis, and Entamoeba spp. J. Microbiol. Methods. 2016;127:68–73. doi: 10.1016/j.mimet.2016.05.020. - DOI - PubMed
-
- Guthrie R., Susi A. A simple phenylalanine method for detecting phenylketonuria in large population of newborn infants. Pediatrics. 1963;32:338–343. - PubMed
-
- GE Healthcare Life Sciences Your Forensic Samples, Our Experience. [(accessed on 1 May 2021)]; Available online: www.gelifesciences.com/forensics.
LinkOut - more resources
Full Text Sources

