The Combination of Cell Cultured Technology and In Silico Model to Inform the Drug Development
- PMID: 34065907
- PMCID: PMC8151315
- DOI: 10.3390/pharmaceutics13050704
The Combination of Cell Cultured Technology and In Silico Model to Inform the Drug Development
Abstract
Human-derived in vitro models can provide high-throughput efficacy and toxicity data without a species gap in drug development. Challenges are still encountered regarding the full utilisation of massive data in clinical settings. The lack of translated methods hinders the reliable prediction of clinical outcomes. Therefore, in this study, in silico models were proposed to tackle these obstacles from in vitro to in vivo translation, and the current major cell culture methods were introduced, such as human-induced pluripotent stem cells (hiPSCs), 3D cells, organoids, and microphysiological systems (MPS). Furthermore, the role and applications of several in silico models were summarised, including the physiologically based pharmacokinetic model (PBPK), pharmacokinetic/pharmacodynamic model (PK/PD), quantitative systems pharmacology model (QSP), and virtual clinical trials. These credible translation cases will provide templates for subsequent in vitro to in vivo translation. We believe that synergising high-quality in vitro data with existing models can better guide drug development and clinical use.
Keywords: human-induced pluripotent stem cells; in vitro to in vivo translation; microphysiological systems; organoid; pharmacokinetic/pharmacodynamic model; physiologically based pharmacokinetic model; quantitative systems pharmacology model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
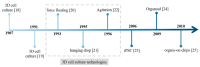
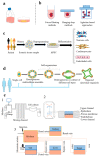
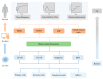
References
-
- Waring M.J., Arrowsmith J., Leach A.R., Leeson P.D., Mandrell S., Owen R.M., Pairaudeau G., Pennie W.D., Pickett S.D., Wang J., et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015;14:475–486. doi: 10.1038/nrd4609. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

