Analytical Validation and Clinical Application of Rapid Serological Tests for SARS-CoV-2 Suitable for Large-Scale Screening
- PMID: 34065954
- PMCID: PMC8151461
- DOI: 10.3390/diagnostics11050869
Analytical Validation and Clinical Application of Rapid Serological Tests for SARS-CoV-2 Suitable for Large-Scale Screening
Abstract
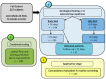
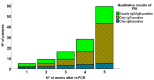
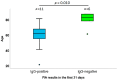
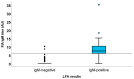
Recently, large-scale screening for COVID-19 has presented a major challenge, limiting timely countermeasures. Therefore, the application of suitable rapid serological tests could provide useful information, however, little evidence regarding their robustness is currently available. In this work, we evaluated and compared the analytical performance of a rapid lateral-flow test (LFA) and a fast semiquantitative fluorescent immunoassay (FIA) for anti-nucleocapsid (anti-NC) antibodies, with the reverse transcriptase real-time PCR assay as the reference. In 222 patients, LFA showed poor sensitivity (55.9%) within two weeks from PCR, while later testing was more reliable (sensitivity of 85.7% and specificity of 93.1%). Moreover, in a subset of 100 patients, FIA showed high sensitivity (89.1%) and specificity (94.1%) after two weeks from PCR. The coupled application for the screening of 183 patients showed satisfactory concordance (K = 0.858). In conclusion, rapid serological tests were largely not useful for early diagnosis, but they showed good performance in later stages of infection. These could be useful for back-tracing and/or to identify potentially immune subjects.
Keywords: COVID-19; IgG; IgM; antibodies; immune response; microsampling; serological test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of two SARS-CoV-2 IgG/IgM Rapid Tests in Capillary Blood Samples.Clin Lab. 2023 Jan 1;69(1). doi: 10.7754/Clin.Lab.2022.220421. Clin Lab. 2023. PMID: 36649507
-
A Highly Sensitive and Specific SARS-CoV-2 Spike- and Nucleoprotein-Based Fluorescent Multiplex Immunoassay (FMIA) to Measure IgG, IgA, and IgM Class Antibodies.Microbiol Spectr. 2021 Dec 22;9(3):e0113121. doi: 10.1128/Spectrum.01131-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34787485 Free PMC article.
-
Recent Advances in the Evaluation of Serological Assays for the Diagnosis of SARS-CoV-2 Infection and COVID-19.Front Public Health. 2021 Feb 18;8:620222. doi: 10.3389/fpubh.2020.620222. eCollection 2020. Front Public Health. 2021. PMID: 33681115 Free PMC article.
-
A systematic and meta-analysis review on the diagnostic accuracy of antibodies in the serological diagnosis of COVID-19.Syst Rev. 2021 May 26;10(1):155. doi: 10.1186/s13643-021-01689-3. Syst Rev. 2021. PMID: 34039423 Free PMC article. Review.
-
Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis.BMJ. 2020 Jul 1;370:m2516. doi: 10.1136/bmj.m2516. BMJ. 2020. PMID: 32611558 Free PMC article.
Cited by
-
Possible Impact of Vitamin D Status and Supplementation on SARS-CoV-2 Infection Risk and COVID-19 Symptoms in a Cohort of Patients with Inflammatory Bowel Disease.Nutrients. 2022 Dec 29;15(1):169. doi: 10.3390/nu15010169. Nutrients. 2022. PMID: 36615826 Free PMC article.
References
-
- ISS COVID-19 Integrated Surveillance: Key National Data. [(accessed on 12 April 2021)]; Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/Infografica_12apr....
-
- WHO . Clinical Management of COVID-19. WHO; Geneva, Switzerland: 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

