Some Dietary Phenolic Compounds Can Activate Thyroid Peroxidase and Inhibit Lipoxygenase-Preliminary Study in the Model Systems
- PMID: 34065957
- PMCID: PMC8151655
- DOI: 10.3390/ijms22105108
Some Dietary Phenolic Compounds Can Activate Thyroid Peroxidase and Inhibit Lipoxygenase-Preliminary Study in the Model Systems
Abstract
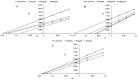
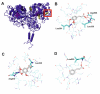
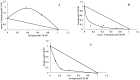
The presented research concerns the triple activity of trans-cinnamic (tCA), ferulic (FA) and syringic acids (SA). They act as thyroid peroxidase (TPO) activators, lipoxygenase (LOX) inhibitors and show antiradical activity. All compounds showed a dose-dependent TPO activatory effect, thus the AC50 value (the concentration resulting in 50% activation) was determined. The tested compounds can be ranked as follows: tCA > FA > SA with AC50 = 0.10, 0.39, 0.69 mM, respectively. Strong synergism was found between FA and SA. The activatory effects of all tested compounds may result from interaction with the TPO allosteric site. It was proposed that conformational change resulting from activator binding to TPO allosteric pocket results from the flexibility of a nearby loop formed by residues Val352-Tyr363. All compounds act as uncompetitive LOX inhibitors. The most effective were tCA and SA, whereas the weakest was FA (IC50 = 0.009 mM and IC50 0.027 mM, respectively). In all cases, an interaction between the inhibitors carboxylic groups and side-chain atoms of Arg102 and Arg139 in an allosteric pocket of LOX was suggested. FA/tCA and FA/SA acted synergistically, whereas tCA/SA demonstrated antagonism. The highest antiradical activity was found in the case of SA (IC50 = 0.22 mM). FA/tCA and tCA/SA acted synergistically, whereas antagonism was found for the SA/FA mixture.
Keywords: antioxidant activity; dietary polyphenols; inhibition; interactions; isobolographic analysis; lipoxygenase (LOX); thyroid peroxidase (TPO).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources.Biomolecules. 2019 Oct 29;9(11):663. doi: 10.3390/biom9110663. Biomolecules. 2019. PMID: 31671724 Free PMC article.
-
Thyroid Peroxidase Activity is Inhibited by Phenolic Compounds-Impact of Interaction.Molecules. 2019 Jul 30;24(15):2766. doi: 10.3390/molecules24152766. Molecules. 2019. PMID: 31366075 Free PMC article.
-
Modelling of Thyroid Peroxidase Reveals Insights into Its Enzyme Function and Autoantigenicity.PLoS One. 2015 Dec 1;10(12):e0142615. doi: 10.1371/journal.pone.0142615. eCollection 2015. PLoS One. 2015. PMID: 26623656 Free PMC article.
-
Development of studies of TPO gene and its application in nuclear medicine.Nucl Med Commun. 2003 Aug;24(8):853-6. doi: 10.1097/01.mnm.0000084582.29433.96. Nucl Med Commun. 2003. PMID: 12869816 Review.
-
A comparison of potency differences among thyroid peroxidase (TPO) inhibitors to induce developmental toxicity and other thyroid gland-linked toxicities in humans and rats.Regul Toxicol Pharmacol. 2016 Oct;80:283-90. doi: 10.1016/j.yrtph.2016.06.019. Epub 2016 Jun 24. Regul Toxicol Pharmacol. 2016. PMID: 27350053 Review.
Cited by
-
The Role of Nutrition on Thyroid Function.Nutrients. 2024 Jul 31;16(15):2496. doi: 10.3390/nu16152496. Nutrients. 2024. PMID: 39125376 Free PMC article. Review.
-
Toxicity profiling and HR-LCMS analysis of Indigofera longiracemosa leaf methanolic extract exhibiting anti-inflammatory activity.3 Biotech. 2025 Jun;15(6):160. doi: 10.1007/s13205-025-04320-7. Epub 2025 May 9. 3 Biotech. 2025. PMID: 40352767
-
Oatmeal and wheat flour as the sources of thyroid peroxidase and proinflammatory enzymes modulators in the prevention of thyroid diseases.Sci Rep. 2025 Jan 9;15(1):1525. doi: 10.1038/s41598-025-85848-9. Sci Rep. 2025. PMID: 39789123 Free PMC article.
-
Cherries with Different Geographical Origins Regulate Neuroprotection in a Photoperiod-Dependent Manner in F344 Rats.Antioxidants (Basel). 2024 Jan 3;13(1):72. doi: 10.3390/antiox13010072. Antioxidants (Basel). 2024. PMID: 38247496 Free PMC article.
-
Classic Phytochemical Antioxidant and Lipoxygenase Inhibitor, Nordihydroguaiaretic Acid, Activates Phospholipase D through Oxidant Signaling and Tyrosine Phosphorylation Leading to Cytotoxicity in Lung Vascular Endothelial Cells.Cell Biochem Biophys. 2023 Jun;81(2):205-229. doi: 10.1007/s12013-023-01128-1. Epub 2023 Feb 23. Cell Biochem Biophys. 2023. PMID: 36820994
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

