Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook
- PMID: 34065960
- PMCID: PMC8150927
- DOI: 10.3390/cancers13102317
Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook
Abstract
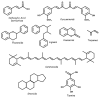
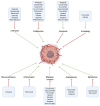
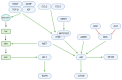
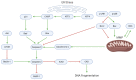
Glioblastoma (GBM) is an aggressive, often fatal astrocyte-derived tumor of the central nervous system. Conventional medical and surgical interventions have greatly improved survival rates; however, tumor heterogeneity, invasiveness, and chemotherapeutic resistance continue to pose clinical challenges. As such, dietary natural substances-an integral component of the lifestyle medicine approach to chronic diseases-are examined as potential chemotherapeutic agents. These heterogenous substances exert anti-GBM effects by upregulating apoptosis and autophagy, inducing cell cycle arrest, interfering with tumor metabolism, and inhibiting proliferation, neuroinflammation, chemoresistance, angiogenesis, and metastasis. Although these beneficial effects are promising, natural substances' efficacy in GBM is constrained by their bioavailability and blood-brain barrier permeability; various chemical formulations are proposed to improve their pharmacological properties. Many of the reviewed substances are available as over-the-counter dietary supplements, underscoring their viability as lifestyle interventions. However, clinical trials remain necessary to substantiate the in vitro and in vivo properties of natural substances.
Keywords: brain cancer; carotenoids; coumarins; flavonoids; glioblastoma; lifestyle medicine; lignans; natural compounds; polyphenols; steroids; tannins; terpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ringel F., Pape H., Sabel M., Krex D., Bock H.C., Misch M., Weyerbrock A., Westermaier T., Senft C., Schucht P., et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18:96–104. doi: 10.1093/neuonc/nov145. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

