PERK Is Critical for Alphavirus Nonstructural Protein Translation
- PMID: 34065980
- PMCID: PMC8151226
- DOI: 10.3390/v13050892
PERK Is Critical for Alphavirus Nonstructural Protein Translation
Retraction in
-
RETRACTED: Dahal et al. PERK Is Critical for Alphavirus Nonstructural Protein Translation. Viruses 2021, 13, 892.Viruses. 2025 Feb 26;17(3):318. doi: 10.3390/v17030318. Viruses. 2025. PMID: 40143380 Free PMC article.
Abstract
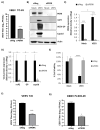
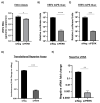
Venezuelan equine encephalitis virus (VEEV) is an alphavirus that causes encephalitis. Previous work indicated that VEEV infection induced early growth response 1 (EGR1) expression, leading to cell death via the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) arm of the unfolded protein response (UPR) pathway. Loss of PERK prevented EGR1 induction and decreased VEEV-induced death. The results presented within show that loss of PERK in human primary astrocytes dramatically reduced VEEV and eastern equine encephalitis virus (EEEV) infectious titers by 4-5 log10. Loss of PERK also suppressed VEEV replication in primary human pericytes and human umbilical vein endothelial cells, but it had no impact on VEEV replication in transformed U87MG and 293T cells. A significant reduction in VEEV RNA levels was observed as early as 3 h post-infection, but viral entry assays indicated that the loss of PERK minimally impacted VEEV entry. In contrast, the loss of PERK resulted in a dramatic reduction in viral nonstructural protein translation and negative-strand viral RNA production. The loss of PERK also reduced the production of Rift Valley fever virus and Zika virus infectious titers. These data indicate that PERK is an essential factor for the translation of alphavirus nonstructural proteins and impacts multiple RNA viruses, making it an exciting target for antiviral development.
Keywords: PERK; Venezuelan equine encephalitis virus (VEEV); alphavirus; eastern equine encephalitis virus (EEEV); translation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Gardner C.L., Burke C.W., Tesfay M.Z., Glass P.J., Klimstra W.B., Ryman K.D. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: Impact of altered cell tropism on pathogenesis. J. Virol. 2008;82:10634–10646. doi: 10.1128/JVI.01323-08. - DOI - PMC - PubMed
-
- Dahal B., Lin S.-C., Carey B.D., Jacobs J.L., Dinman J.D., van Hoek M.L., Adams A.A., Kehn-Hall K. EGR1 upregulation following Venezuelan equine encephalitis virus infection is regulated by ERK and PERK pathways contributing to cell death. Virology. 2019 doi: 10.1016/j.virol.2019.10.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

