A [68Ga]Ga-DOTANOC PET/CT Radiomic Model for Non-Invasive Prediction of Tumour Grade in Pancreatic Neuroendocrine Tumours
- PMID: 34065981
- PMCID: PMC8150289
- DOI: 10.3390/diagnostics11050870
A [68Ga]Ga-DOTANOC PET/CT Radiomic Model for Non-Invasive Prediction of Tumour Grade in Pancreatic Neuroendocrine Tumours
Abstract
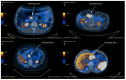
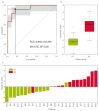
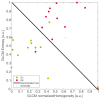
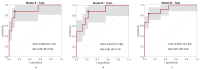
Predicting grade 1 (G1) and 2 (G2) primary pancreatic neuroendocrine tumour (panNET) is crucial to foresee panNET clinical behaviour. Fifty-one patients with G1-G2 primary panNET demonstrated by pre-surgical [68Ga]Ga-DOTANOC PET/CT and diagnostic conventional imaging were grouped according to the tumour grade assessment method: histology on the whole excised primary lesion (HS) or biopsy (BS). First-order and second-order radiomic features (RFs) were computed from SUV maps for the whole tumour volume on HS. The RFs showing the lowest p-values and the highest area under the curve (AUC) were selected. Three radiomic models were assessed: A (trained on HS, validated on BS), B (trained on BS, validated on HS), and C (using the cross-validation on the whole dataset). The second-order normalized homogeneity and entropy was the most effective RFs couple predicting G2 and G1. The best performance was achieved by model A (test AUC = 0.90, sensitivity = 0.88, specificity = 0.89), followed by model C (median test AUC = 0.87, sensitivity = 0.83, specificity = 0.82). Model B performed worse. Using HS to train a radiomic model leads to the best prediction, although a "hybrid" (HS+BS) population performs better than biopsy-only. The non-invasive prediction of panNET grading may be especially useful in lesions not amenable to biopsy while [68Ga]Ga-DOTANOC heterogeneity might recommend FDG PET/CT.
Keywords: [68Ga]Ga-DOTANOC; biomarker; machine learning; pancreatic neuroendocrine tumour; standardized uptake value.
Conflict of interest statement
Alessandro Bevilacqua, Diletta Calabrò, Silvia Malavasi, Claudio Ricci, Riccardo Casadei, and Serena Baiocco have no financial and/or competing interest. Davide Campana is a member of the executive board of ITANET; Stefano Fanti has received speaker honorarium from Bayer, Astellas, GE, Blue Earth, ANMI and is advisor for Bayer, Astellas, GE, Blue Earth, ANMI; Valentina Ambrosini has received speaker honorarium from AAA and ESMIT and is member of the advisory board of ENETS and of the scientific board of ITANET and of the Faculty staff of ESMO.
Figures


References
-
- Perren A., Couvelard A., Scoazec J.-Y., Costa F., Borbath I., Delle Fave G., Gorbounova V., Gross D., Grossma A., Jense R.T., et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology. 2017;105:196–200. doi: 10.1159/000457956. - DOI - PubMed
-
- Miederer M., Seidl S., Buck A., Scheidhauer K., Wester H.-J., Schwaiger M., Perren A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:48–52. doi: 10.1007/s00259-008-0944-5. - DOI - PubMed
-
- Campana D., Ambrosini V., Pezzilli R., Fanti S., Labate A.M.M., Santini D., Ceccarelli C., Nori F., Franchi R., Corinaldesi R., et al. Standardized uptake values of (68)Ga-DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2010;51:353–359. - PubMed
LinkOut - more resources
Full Text Sources

