Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila
- PMID: 34065985
- PMCID: PMC8151258
- DOI: 10.3390/v13050894
Physical and Chemical Barriers in the Larval Midgut Confer Developmental Resistance to Virus Infection in Drosophila
Abstract
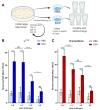
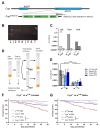
Insects can become lethally infected by the oral intake of a number of insect-specific viruses. Virus infection commonly occurs in larvae, given their active feeding behaviour; however, older larvae often become resistant to oral viral infections. To investigate mechanisms that contribute to resistance throughout the larval development, we orally challenged Drosophila larvae at different stages of their development with Drosophila C virus (DCV, Dicistroviridae). Here, we showed that DCV-induced mortality is highest when infection initiates early in larval development and decreases the later in development the infection occurs. We then evaluated the peritrophic matrix as an antiviral barrier within the gut using a Crystallin-deficient fly line (Crys-/-), whose PM is weakened and becomes more permeable to DCV-sized particles as the larva ages. This phenotype correlated with increasing mortality the later in development oral challenge occurred. Lastly, we tested in vitro the infectivity of DCV after incubation at pH conditions that may occur in the midgut. DCV virions were stable in a pH range between 3.0 and 10.5, but their infectivity decreased at least 100-fold below (1.0) and above (12.0) this range. We did not observe such acidic conditions in recently hatched larvae. We hypothesise that, in Drosophila larvae, the PM is essential for containing ingested virions separated from the gut epithelium, while highly acidic conditions inactivate the majority of the virions as they transit.
Keywords: Drosophila; antiviral mechanisms; dicistrovirus; gut pH; larval development; midgut; peritrophic matrix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

