Cardiovascular Stents: A Review of Past, Current, and Emerging Devices
- PMID: 34065986
- PMCID: PMC8151529
- DOI: 10.3390/ma14102498
Cardiovascular Stents: A Review of Past, Current, and Emerging Devices
Abstract
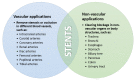
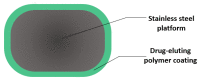
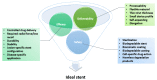
One of the leading causes of morbidity and mortality worldwide is coronary artery disease, a condition characterized by the narrowing of the artery due to plaque deposits. The standard of care for treating this disease is the introduction of a stent at the lesion site. This life-saving tubular device ensures vessel support, keeping the blood-flow path open so that the cardiac muscle receives its vital nutrients and oxygen supply. Several generations of stents have been iteratively developed towards improving patient outcomes and diminishing adverse side effects following the implanting procedure. Moving from bare-metal stents to drug-eluting stents, and recently reaching bioresorbable stents, this research field is under continuous development. To keep up with how stent technology has advanced in the past few decades, this paper reviews the evolution of these devices, focusing on how they can be further optimized towards creating an ideal vascular scaffold.
Keywords: cardiovascular stents; stent optimization; stent platform materials; surface functionalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kulkarni P., Rawtani D., Kumar M., Lahoti S.R. Cardiovascular drug delivery: A review on the recent advancements in nanocarrier based drug delivery with a brief emphasis on the novel use of magnetoliposomes and extracellular vesicles and ongoing clinical trial research. J. Drug Deliv. Sci. Technol. 2020;60:102029. doi: 10.1016/j.jddst.2020.102029. - DOI
-
- Synthesis of new azaindeno-acetonitrile derivative with inotropic activity against heart failure model. Biointerface Res. Appl. Chem. 2019;9:4598–4604. doi: 10.33263/BRIAC96.598604. - DOI
-
- Microscopic and submicroscopic structure of the heart atria and auricles in condition of the experimental thermal trauma. Biointerface Res. Appl. Chem. 2020;10:5237–5242. doi: 10.33263/BRIAC102.237242. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

