Scalable, Micro-Neutralization Assay for Assessment of SARS-CoV-2 (COVID-19) Virus-Neutralizing Antibodies in Human Clinical Samples
- PMID: 34065987
- PMCID: PMC8151879
- DOI: 10.3390/v13050893
Scalable, Micro-Neutralization Assay for Assessment of SARS-CoV-2 (COVID-19) Virus-Neutralizing Antibodies in Human Clinical Samples
Abstract
As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic expanded, it was clear that effective testing for the presence of neutralizing antibodies in the blood of convalescent patients would be critical for development of plasma-based therapeutic approaches. To address the need for a high-quality neutralization assay against SARS-CoV-2, a previously established fluorescence reduction neutralization assay (FRNA) against Middle East respiratory syndrome coronavirus (MERS-CoV) was modified and optimized. The SARS-CoV-2 FRNA provides a quantitative assessment of a large number of infected cells through use of a high-content imaging system. Because of this approach, and the fact that it does not involve subjective interpretation, this assay is more efficient and more accurate than other neutralization assays. In addition, the ability to set robust acceptance criteria for individual plates and specific test wells provided further rigor to this assay. Such agile adaptability avails use with multiple virus variants. By February 2021, the SARS-CoV-2 FRNA had been used to screen over 5000 samples, including acute and convalescent plasma or serum samples and therapeutic antibody treatments, for SARS-CoV-2 neutralizing titers.
Keywords: COVID; COVID-19; SARS-CoV; SARS-CoV-2; antibodies; coronavirus; diagnosis; neutralization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
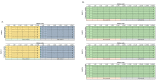
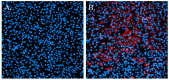
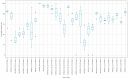
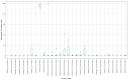
Update of
-
Scalable, Micro-Neutralization Assay for Qualitative Assessment of SARS-CoV-2 (COVID-19) Virus-Neutralizing Antibodies in Human Clinical Samples.bioRxiv [Preprint]. 2021 Mar 5:2021.03.05.434152. doi: 10.1101/2021.03.05.434152. bioRxiv. 2021. Update in: Viruses. 2021 May 12;13(5):893. doi: 10.3390/v13050893. PMID: 33688658 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

