Galangin Resolves Cardiometabolic Disorders through Modulation of AdipoR1, COX-2, and NF-κB Expression in Rats Fed a High-Fat Diet
- PMID: 34066039
- PMCID: PMC8150752
- DOI: 10.3390/antiox10050769
Galangin Resolves Cardiometabolic Disorders through Modulation of AdipoR1, COX-2, and NF-κB Expression in Rats Fed a High-Fat Diet
Abstract
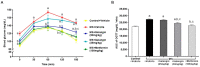
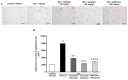
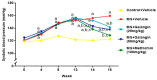
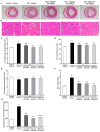
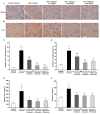
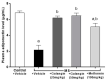
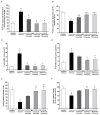
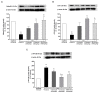
Galangin is a natural flavonoid. In this study, we evaluated whether galangin could alleviate signs of metabolic syndrome (MS) and cardiac abnormalities in rats receiving a high-fat (HF) diet. Male Sprague-Dawley rats were given an HF diet plus 15% fructose for four months, and they were fed with galangin (25 or 50 mg/kg), metformin (100 mg/kg), or a vehicle for the last four weeks. The MS rats exhibited signs of MS, hypertrophy of adipocytes, impaired liver function, and cardiac dysfunction and remodeling. These abnormalities were alleviated by galangin (p < 0.05). Interleukin-6 and tumor necrosis factor-α concentrations and expression were high in the plasma and cardiac tissue in the MS rats, and these markers were suppressed by galangin (p < 0.05). These treatments also alleviated the low levels of adiponectin and oxidative stress induced by an HF diet in rats. The downregulation of adiponectin receptor 1 (AdipoR1) and cyclooxygenase-2 (COX-2) and the upregulation of nuclear factor kappa B (NF-κB) expression were recovered in the galangin-treated groups. Metformin produced similar effects to galangin. In conclusion, galangin reduced cardiometabolic disorders in MS rats. These effects might be linked to the suppression of inflammation and oxidative stress and the restoration of AdipoR1, COX-2, and NF-κB expression.
Keywords: adiponectin; cardiac function; galangin; inflammation; metabolic syndrome; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitigation effect of galangin against aortic dysfunction and hypertrophy in rats with metabolic syndrome.Heliyon. 2023 May 19;9(5):e16500. doi: 10.1016/j.heliyon.2023.e16500. eCollection 2023 May. Heliyon. 2023. PMID: 37251824 Free PMC article.
-
Diosmetin attenuates metabolic syndrome and left ventricular alterations via the suppression of angiotensin II/AT1 receptor/gp91phox/p-NF-κB protein expression in high-fat diet fed rats.Food Funct. 2021 Mar 1;12(4):1469-1481. doi: 10.1039/d0fo02744h. Food Funct. 2021. PMID: 33449987
-
Galangin alleviates vascular dysfunction and remodelling through modulation of the TNF-R1, p-NF-κB and VCAM-1 pathways in hypertensive rats.Life Sci. 2021 Nov 15;285:119965. doi: 10.1016/j.lfs.2021.119965. Epub 2021 Sep 17. Life Sci. 2021. PMID: 34543638
-
Hesperidin alleviates vascular dysfunction and remodelling in high-fat/high-fructose diet-fed rats by modulating oxidative stress, inflammation, AdipoR1, and eNOS expression.Tissue Cell. 2022 Oct;78:101901. doi: 10.1016/j.tice.2022.101901. Epub 2022 Aug 19. Tissue Cell. 2022. PMID: 36007378
-
Effect of galangin supplementation on oxidative damage and inflammatory changes in fructose-fed rat liver.Chem Biol Interact. 2011 Sep 5;193(2):141-8. doi: 10.1016/j.cbi.2011.06.003. Epub 2011 Jun 25. Chem Biol Interact. 2011. PMID: 21708140
Cited by
-
Tangeretin Unravels Metabolic Dysfunction-Associated Fatty Liver Disease in Rats by Enhancing the IRS/Akt Pathway.Life (Basel). 2025 Mar 18;15(3):491. doi: 10.3390/life15030491. Life (Basel). 2025. PMID: 40141836 Free PMC article.
-
Galangin Attenuates Myocardial Ischemic Reperfusion-Induced Ferroptosis by Targeting Nrf2/Gpx4 Signaling Pathway.Drug Des Devel Ther. 2023 Aug 22;17:2495-2511. doi: 10.2147/DDDT.S409232. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37637264 Free PMC article.
-
Cardiovascular complications are resolved by tuna protein hydrolysate supplementation in rats fed with a high-fat diet.Sci Rep. 2023 Jul 28;13(1):12280. doi: 10.1038/s41598-023-39538-z. Sci Rep. 2023. PMID: 37507421 Free PMC article.
-
Diosmetin Ameliorates Vascular Dysfunction and Remodeling by Modulation of Nrf2/HO-1 and p-JNK/p-NF-κB Expression in Hypertensive Rats.Antioxidants (Basel). 2021 Sep 17;10(9):1487. doi: 10.3390/antiox10091487. Antioxidants (Basel). 2021. PMID: 34573119 Free PMC article.
-
Mitigation effect of galangin against aortic dysfunction and hypertrophy in rats with metabolic syndrome.Heliyon. 2023 May 19;9(5):e16500. doi: 10.1016/j.heliyon.2023.e16500. eCollection 2023 May. Heliyon. 2023. PMID: 37251824 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

