Transient Global Ischemia-Induced Brain Inflammatory Cascades Attenuated by Targeted Temperature Management
- PMID: 34066051
- PMCID: PMC8151768
- DOI: 10.3390/ijms22105114
Transient Global Ischemia-Induced Brain Inflammatory Cascades Attenuated by Targeted Temperature Management
Abstract
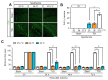
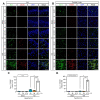
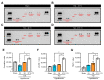
Sudden cardiac arrest leads to a significantly increased risk of severe neurological impairment and higher mortality rates in survivors due to global brain tissue injury caused by prolonged whole-body ischemia and reperfusion. The brain undergoes various deleterious cascading events. Among these damaging mechanisms, neuroinflammation plays an especially crucial role in the exacerbation of brain damage. Clinical guidelines indicate that 33 °C and 36 °C are both beneficial for targeted temperature management (TTM) after cardiac arrest. To clarify the mechanistic relationship between TTM and inflammation in transient global ischemia (TGI) and determine whether 36 °C produces a neuroprotective effect comparable to 33 °C, we performed an experiment using a rat model. We found that TTM at 36 °C and at 33 °C attenuated neuronal cell death and apoptosis, with significant improvements in behavioral function that lasted for up to 72 h. TTM at 33 °C and 36 °C suppressed the propagation of inflammation including the release of high mobility group box 1 from damaged cells, the activation and polarization of the microglia, and the excessive release of activated microglia-induced inflammatory cytokines. There were equal neuroprotective effects for TTM at 36 °C and 33 °C. In addition, hypothermic complications and should be considered safe and effective after cardiac arrest.
Keywords: apoptosis; high mobility box protein 1; inflammation; microglia; post cardiac arrest care; targeted temperature management.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Donnino M.W., Andersen L.W., Berg K.M., Reynolds J.C., Nolan J.P., Morley P.T., Lang E., Cocchi M.N., Xanthos T., Callaway C.W., et al. Temperature management after cardiac arrest: An advisory statement by the advanced life support task force of the international liaison committee on resuscitation and the american heart association emergency cardiovascular care committee and the council on cardiopulmonary, critical care, perioperative and resuscitation. Circulation. 2015;132:2448–2456. doi: 10.1161/cir.0000000000000313. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

