Utility and Mechanism of SHetA2 and Paclitaxel for Treatment of Endometrial Cancer
- PMID: 34066052
- PMCID: PMC8150795
- DOI: 10.3390/cancers13102322
Utility and Mechanism of SHetA2 and Paclitaxel for Treatment of Endometrial Cancer
Abstract
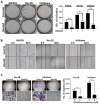
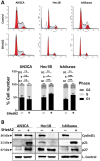
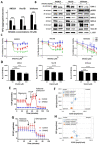
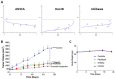
Endometrial cancer patients with advanced disease or high recurrence risk are treated with chemotherapy. Our objective was to evaluate the utility and mechanism of a novel drug, SHetA2, alone and in combination with paclitaxel, in endometrial cancer. SHetA2 targets the HSPA chaperone proteins, Grp78, hsc70, and mortalin, which have high mutation rates in endometrial cancer. SHetA2 effects on cancerous phenotypes, mitochondria, metabolism, protein expression, mortalin/client protein complexes, and cell death were evaluated in AN3CA, Hec13b, and Ishikawa endometrial cancer cell lines, and on growth of Ishikawa xenografts. In all three cell lines, SHetA2 inhibited anchorage-independent growth, migration, invasion, and ATP production, and induced G1 cell cycle arrest, mitochondrial damage, and caspase- and apoptosis inducing factor (AIF)-mediated apoptosis. These effects were associated with altered levels of proteins involved in cell cycle regulation, mitochondrial function, protein synthesis, endoplasmic reticulum stress, and metabolism; disruption of mortalin complexes with mitochondrial and metabolism proteins; and inhibition of oxidative phosphorylation and glycolysis. SHetA2 and paclitaxel exhibited synergistic combination indices in all cell lines and exerted greater xenograft tumor growth inhibition than either drug alone. SHetA2 is active against endometrial cancer cell lines in culture and in vivo and acts synergistically with paclitaxel.
Keywords: SHetA2; apoptosis inducing factor; cell cycle arrest; endometrial cancer; glycolysis; metabolism; mitochondria; oxidative phosphorylation; paclitaxel; xenograft.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cancer Facts and Figures. [(accessed on 1 April 2021)]; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- Aune D., Navarro Rosenblatt D.A., Chan D.S., Vingeliene S., Abar L., Vieira A.R., Greenwood D.C., Bandera E.V., Norat T. Anthropometric factors and endometrial cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Ann. Oncol. 2015;26:1635–1648. doi: 10.1093/annonc/mdv142. - DOI - PubMed
-
- Arem H., Pfeiffer R.M., Moore S.C., Irwin M.L., LaMonte M.J., Sarto G.E., Nassir R., Luo J., Chlebowski R.T., Brinton L.A., et al. Post-diagnosis body mass index and mortality among women diagnosed with endometrial cancer: Results from the Women’s Health Initiative. PLoS ONE. 2017;12:e0171250. doi: 10.1371/journal.pone.0171250. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

