Overview of Cellular Immunotherapies within Transfusion Medicine for the Treatment of Malignant Diseases
- PMID: 34066067
- PMCID: PMC8151282
- DOI: 10.3390/ijms22105120
Overview of Cellular Immunotherapies within Transfusion Medicine for the Treatment of Malignant Diseases
Abstract
Over the years, transfusion medicine has developed into a broad, multidisciplinary field that covers different clinical patient services such as apheresis technology and the development of stem cell transplantation. Recently, the discipline has found a niche in development and production of advanced therapy medicinal products (ATMPs) for immunotherapy and regenerative medicine purposes. In clinical trials, cell-based immunotherapies have shown encouraging results in the treatment of multiple cancers and autoimmune diseases. However, there are many parameters such as safety, a high level of specificity, and long-lasting efficacy that still need to be optimized to maximize the potential of cell-based immunotherapies. Thus, only a few have gained FDA approval, while the majority of them are studied in the context of investigator-initiated trials (IITs), where modern, academically oriented transfusion centers can play an important role. In this review, we summarize existing and contemporary cellular immunotherapies, which are already a part of modern transfusion medicine or are likely to become so in the future.
Keywords: cancer; cell-based immunotherapy; transfusion medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
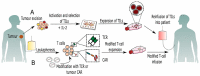
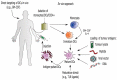
References
-
- Prigent A., Maillard N., Absi L., Aloui C., Cognasse F., Laradi S., Mariat C., Garraud O. From Donor to Recipient: Current Questions Relating to Humoral Alloimmunization. Antibodies. 2014;3:130–152. doi: 10.3390/antib3010130. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

