Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage
- PMID: 34066077
- PMCID: PMC8151616
- DOI: 10.3390/cells10051180
Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage
Abstract
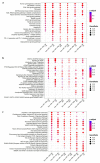
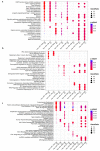
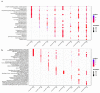
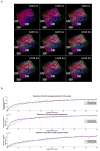
The study of the miRNA cargo embedded in extracellular vesicles (EVs) released from adipose-derived mesenchymal stromal cells (ASC) preconditioned with IL-1β, an inflammatory stimulus driving osteoarthritis (OA), along with EVs-cartilage dynamic interaction represent poorly explored fields and are the purpose of the present research. ASCs were isolated from subcutaneous adipose tissue and EVs collected by ultracentrifugation. Shuttled miRNAs were scored by high-throughput screening and analyzed through bioinformatics approach that predicted the potentially modulated OA-related pathways. Fluorescently labeled EVs incorporation into OA cartilage explants was followed in vitro by time-lapse coherent anti-Stokes Raman scattering; second harmonic generation and two-photon excited fluorescence. After IL-1β preconditioning, 7 miRNA were up-regulated, 4 down-regulated, 37 activated and 17 silenced. Bioinformatics allowed to identify miRNAs and target genes mainly involved in Wnt, Notch, TGFβ and Indian hedgehog (IHH) pathways, cartilage homeostasis, immune/inflammatory responses, cell senescence and autophagy. As well, ASC-EVs steadily diffuse in cartilage cells and matrix, reaching a plateau 16 h after administration. Overall, ASCs preconditioned with IL-1β allows secretion of EVs embedded with a chondro-protective miRNA cargo, able to fast penetrate in collagen-rich areas of cartilage with tissue saturation in a day. Further functional studies exploring the EVs dose-effects are needed to achieve clinical relevance.
Keywords: adipose-derived mesenchymal stromal cells; cartilage; extracellular vesicles; interleukin 1 beta; miRNA; nonlinear optical microscopy; osteoarthritis; time-lapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- De Luca P., Kouroupis D., Viganò M., Perucca-Orfei C., Kaplan L., Zagra L., de Girolamo L., Correa D., Colombini A. Human Diseased Articular Cartilage Con-tains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019;8:423. doi: 10.3390/jcm8040423. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

