Determination of Folic Acid Using Biosensors-A Short Review of Recent Progress
- PMID: 34066085
- PMCID: PMC8150745
- DOI: 10.3390/s21103360
Determination of Folic Acid Using Biosensors-A Short Review of Recent Progress
Abstract
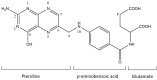
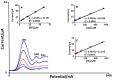
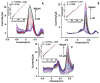
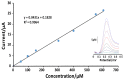
Folic acid (FA) is the synthetic surrogate of the essential B vitamin folate, alternatively named folacin, pteroylglutamic acid or vitamin B9. FA is an electroactive compound that helps our body to create and keep our cells healthy: it acts as the main character in a variety of synthetic biological reactions such as the synthesis of purines, pyrimidine (thus being indirectly implied in DNA synthesis), fixing and methylation of DNA. Therefore, physiological folate deficiency may be responsible for severe degenerative conditions, including neural tube defects in developing embryos and megaloblastic anaemia at any age. Moreover, being a water-soluble molecule, it is constantly lost and has to be reintegrated daily; for this reason, FA supplements and food fortification are, nowadays, extremely diffused and well-established practices. Consequently, accurate, reliable and precise analytical techniques are needed to exactly determine FA concentration in various media. Thus, the aim of this review is to report on research papers of the past 5 years (2016-2020) dealing with rapid and low-cost electrochemical determination of FA in food or biological fluid samples.
Keywords: analytical methods; electrochemical tools; folic acid; real samples.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Andlid T.A., D’Aimmo M.R., Jastrebova J. the Bifidobacteria and Related Organisms. Elsevier; Amsterdam, The Netherlands: 2018. Folate and bifidobacteria; pp. 195–212.
-
- Talwar G.P. Textbook of Biochemistry, Biotechnology, Allied and Molecular Medicine. 4th ed. Prentice-Hall of India; New Delhi, India: 2015. Place of Publication Not Identified.
-
- Bandžuchová L., Šelešovská R., Navrátil T., Chýlková J. Electrochemical behavior of folic acid on mercury meniscus modified silver solid amalgam electrode. Electrochim. Acta. 2011;56:2411–2419. doi: 10.1016/j.electacta.2010.10.090. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

