Minimalistic In Vitro Culture to Drive Human Naive B Cell Differentiation into Antibody-Secreting Cells
- PMID: 34066151
- PMCID: PMC8151070
- DOI: 10.3390/cells10051183
Minimalistic In Vitro Culture to Drive Human Naive B Cell Differentiation into Antibody-Secreting Cells
Abstract
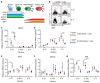
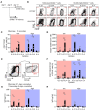
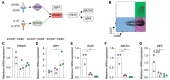
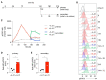
High-affinity antibody-secreting cells (ASC) arise from terminal differentiation of B-cells after coordinated interactions with T follicular helper (Tfh) cells in germinal centers (GC). Elucidation of cues promoting human naive B-cells to progress into ASCs is challenging, as this process is notoriously difficult to induce in vitro while maintaining enough cell numbers to investigate the differentiation route(s). Here, we describe a minimalistic in vitro culture system that supports efficient differentiation of human naive B-cells into antibody-secreting cells. Upon initial stimulations, the interplay between level of CD40 costimulation and the Tfh cell-associated cytokines IL-21 and IL-4 determined the magnitude of B-cell expansion, immunoglobulin class-switching and expression of ASC regulator PRDM1. In contrast, the B-cell-specific transcriptional program was maintained, and efficient ASC formation was hampered. Renewed CD40 costimulation and Tfh cytokines exposure induced rapid secondary STAT3 signaling and extensive ASC differentiation, accompanied by repression of B-cell identity factors PAX5, BACH2 and IRF8 and further induction of PRDM1. Our work shows that, like in vivo, renewed CD40L costimulation also induces efficient terminal ASC differentiation after initial B-cell expansion in vitro. This culture system for efficient differentiation of human naive B-cells into ASCs, while also maintaining high cell numbers, may form an important tool in dissecting human naive B-cell differentiation, thereby enabling identification of novel transcriptional regulators and biomarkers for desired and detrimental antibody formation in humans.
Keywords: cell differentiation; costimulatory molecules; cytokines; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Laffitte E., Skaria M., Jaunin F., Tamm K., Saurat J.-H., Favre B., Borradori L. Autoantibodies to the extracellular and intracellular domain of bullous pemphigoid 180, the putative key autoantigen in bullous pemphigoid, belong predominantly to the IgG1 and IgG4 subclasses. Br. J. Dermatol. 2001;144:760–768. doi: 10.1046/j.1365-2133.2001.04130.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

