CLCA1 Regulates Airway Mucus Production and Ion Secretion Through TMEM16A
- PMID: 34066250
- PMCID: PMC8151571
- DOI: 10.3390/ijms22105133
CLCA1 Regulates Airway Mucus Production and Ion Secretion Through TMEM16A
Abstract
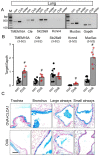
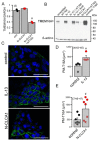
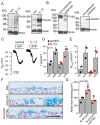
TMEM16A, a Ca2+-activated chloride channel (CaCC), and its regulator, CLCA1, are associated with inflammatory airway disease and goblet cell metaplasia. CLCA1 is a secreted protein with protease activity that was demonstrated to enhance membrane expression of TMEM16A. Expression of CLCA1 is particularly enhanced in goblet cell metaplasia and is associated with various lung diseases. However, mice lacking expression of CLCA1 showed the same degree of mucous cell metaplasia and airway hyperreactivity as asthmatic wild-type mice. To gain more insight into the role of CLCA1, we applied secreted N-CLCA1, produced in vitro, to mice in vivo using intratracheal instillation. We observed no obvious upregulation of TMEM16A membrane expression by CLCA1 and no differences in ATP-induced short circuit currents (Iscs). However, intraluminal mucus accumulation was observed by treatment with N-CLCA1 that was not seen in control animals. The effects of N-CLCA1 were augmented in ovalbumin-sensitized mice. Mucus production induced by N-CLCA1 in polarized BCi-NS1 human airway epithelial cells was dependent on TMEM16A expression. IL-13 upregulated expression of CLCA1 and enhanced mucus production, however, without enhancing purinergic activation of Isc. In contrast to polarized airway epithelial cells and mouse airways, which express very low levels of TMEM16A, nonpolarized airway cells express large amounts of TMEM16A protein and show strong CaCC. The present data show an only limited contribution of TMEM16A to airway ion secretion but suggest a significant role of both CLCA1 and TMEM16A for airway mucus secretion.
Keywords: CLCA1; KCNN4; MUC5AC; TMEM16A; airway epithelium; anoctamin 1; mucus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
TMEM16A Mediates Mucus Production in Human Airway Epithelial Cells.Am J Respir Cell Mol Biol. 2021 Jan;64(1):50-58. doi: 10.1165/rcmb.2019-0442OC. Am J Respir Cell Mol Biol. 2021. PMID: 33026825
-
TMEM16A is indispensable for basal mucus secretion in airways and intestine.FASEB J. 2019 Mar;33(3):4502-4512. doi: 10.1096/fj.201801333RRR. Epub 2018 Dec 26. FASEB J. 2019. PMID: 30586313
-
Mucus Release and Airway Constriction by TMEM16A May Worsen Pathology in Inflammatory Lung Disease.Int J Mol Sci. 2021 Jul 22;22(15):7852. doi: 10.3390/ijms22157852. Int J Mol Sci. 2021. PMID: 34360618 Free PMC article.
-
CLCA1 and TMEM16A: the link towards a potential cure for airway diseases.Expert Rev Respir Med. 2015 Oct;9(5):503-6. doi: 10.1586/17476348.2015.1081064. Epub 2015 Aug 20. Expert Rev Respir Med. 2015. PMID: 26296094 Free PMC article. Review.
-
Anoctamin pharmacology.Cell Calcium. 2024 Jul;121:102905. doi: 10.1016/j.ceca.2024.102905. Epub 2024 May 10. Cell Calcium. 2024. PMID: 38788257 Review.
Cited by
-
Nutritional Support of Chronic Obstructive Pulmonary Disease.Nutrients. 2025 Mar 26;17(7):1149. doi: 10.3390/nu17071149. Nutrients. 2025. PMID: 40218907 Free PMC article. Review.
-
Conjunctival Fluid Secretion Impairment via CaCC-CFTR Dysfunction Is the Key Mechanism in Environmental Dry Eye.Int J Mol Sci. 2022 Nov 19;23(22):14399. doi: 10.3390/ijms232214399. Int J Mol Sci. 2022. PMID: 36430877 Free PMC article.
-
Serotonin-2 Receptor Agonists Produce Anti-inflammatory Effects through Functionally Selective Mechanisms That Involve the Suppression of Disease-Induced Arginase 1 Expression.ACS Pharmacol Transl Sci. 2024 Jan 25;7(2):478-492. doi: 10.1021/acsptsci.3c00297. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357283 Free PMC article.
-
Pathogenic Relationships in Cystic Fibrosis and Renal Diseases: CFTR, SLC26A9 and Anoctamins.Int J Mol Sci. 2023 Aug 26;24(17):13278. doi: 10.3390/ijms241713278. Int J Mol Sci. 2023. PMID: 37686084 Free PMC article. Review.
-
Polymodal Control of TMEM16x Channels and Scramblases.Int J Mol Sci. 2022 Jan 29;23(3):1580. doi: 10.3390/ijms23031580. Int J Mol Sci. 2022. PMID: 35163502 Free PMC article. Review.
References
-
- Huang F., Zhang H., Wu M., Yang H., Kudo M., Peters C.J., Woodruff P.G., Solberg O.D., Donne M.L., Huang X., et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA. 2012;109:16354–16359. doi: 10.1073/pnas.1214596109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

