Differential Expression Profiles of Cell-to-Matrix-Related Molecules in Adrenal Cortical Tumors: Diagnostic and Prognostic Implications
- PMID: 34066306
- PMCID: PMC8148197
- DOI: 10.3390/jpm11050378
Differential Expression Profiles of Cell-to-Matrix-Related Molecules in Adrenal Cortical Tumors: Diagnostic and Prognostic Implications
Abstract
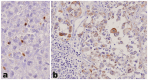
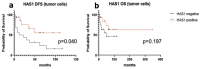
The molecular mechanisms of adrenocortical carcinoma development are incompletely defined. De-regulation of cellular-to-extracellular matrix interactions and angiogenesis appear among mechanisms associated to the malignant phenotype. Our aim was to investigate, employing PCR-based array profiling, 157 molecules involved in cell-to-matrix interactions and angiogenesis in a frozen series of 6 benign and 6 malignant adrenocortical neoplasms, to identify novel pathogenetic markers. In 14 genes, a significant dysregulation was detected in adrenocortical carcinomas as compared to adenomas, most of them being downregulated. Three exceptions-hyaluronan synthase 1 (HAS-1), laminin α3 and osteopontin genes-demonstrated an increased expression in adrenocortical carcinomas of 4.46, 4.23 and 20.32-fold, respectively, and were validated by immunohistochemistry on a series of paraffin-embedded tissues, including 20 adenomas and 73 carcinomas. Osteopontin protein, absent in all adenomas, was expressed in a carcinoma subset (25/73) (p = 0.0022). Laminin α3 and HAS-1 were mostly expressed in smooth muscle and endothelial cells of the vascular network of both benign and malignant adrenocortical tumors. HAS-1 was also detected in tumor cells, with a more intense pattern in carcinomas. In this group, strong expression was significantly associated with more favorable clinicopathological features. These data demonstrate that cell-to-matrix interactions are specifically altered in adrenocortical carcinoma and identify osteopontin and HAS-1 as novel potential diagnostic and prognostic biomarkers, respectively, in adrenal cortical tumors.
Keywords: adrenal cortex; angiogenesis; carcinoma; gene expression; hyaluronan synthase 1; osteopontin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
N-cadherin expression in adrenal tumors: upregulation in malignant pheochromocytoma and downregulation in adrenocortical carcinoma.Endocr Pathol. 2002 Summer;13(2):99-110. doi: 10.1385/ep:13:2:099. Endocr Pathol. 2002. PMID: 12165657
-
Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling.Arch Surg. 2008 Sep;143(9):841-6; discussion 846. doi: 10.1001/archsurg.143.9.841. Arch Surg. 2008. PMID: 18794420
-
Pathologic features and expression of insulin-like growth factor-2 in adrenocortical neoplasms.Endocr Pathol. 2001 Winter;12(4):429-35. doi: 10.1385/ep:12:4:429. Endocr Pathol. 2001. PMID: 11949624
-
Diagnostic and prognostic features in adrenocortical carcinoma: a single institution case series and review of the literature.World J Surg Oncol. 2015 Mar 24;13:117. doi: 10.1186/s12957-015-0527-4. World J Surg Oncol. 2015. PMID: 25889798 Free PMC article. Review.
-
Advances in understanding the molecular underpinnings of adrenocortical tumors.Curr Opin Oncol. 2018 Jan;30(1):16-22. doi: 10.1097/CCO.0000000000000415. Curr Opin Oncol. 2018. PMID: 29028646 Review.
Cited by
-
Effects of in utero exposure to Δ-9-tetrahydrocannabinol on cardiac extracellular matrix expression and vascular transcriptome in rhesus macaques.Am J Physiol Heart Circ Physiol. 2024 Sep 1;327(3):H701-H714. doi: 10.1152/ajpheart.00181.2024. Epub 2024 Jul 19. Am J Physiol Heart Circ Physiol. 2024. PMID: 39028280
-
Special Issue: Present and Future of Personalised Medicine for Endocrine Cancers.J Pers Med. 2022 Apr 29;12(5):710. doi: 10.3390/jpm12050710. J Pers Med. 2022. PMID: 35629133 Free PMC article.
References
-
- Lloyd R.V., Osamura R.Y., Klöppel G., Rosai J., editors. WHO Classification of Tumours of Endocrine Organs. Vol. 10. IARC (International Agency for Research on Cancer), Scientific Publications; Lyon, France: 2017. pp. 163–173.
-
- Giordano T.J., Berney D., de Krijger R.R., Erickson L., Fassnacht M., Mete O., Papathomas T., Papotti M., Sasano H., Thompson L.D.R., et al. Data set for reporting of carcinoma of the adrenal cortex: Explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting. Hum. Pathol. 2020 doi: 10.1016/j.humpath.2020.10.001. - DOI - PubMed
-
- Duregon E., Fassina A., Volante M., Nesi G., Santi R., Gatti G., Cappellesso R., Dalino Ciaramella P., Ventura L., Gambacorta M., et al. The reticulin algorithm for adrenocortical tumor diagnosis: A multicentric validation study on 245 unpublished cases. Am. J. Surg. Pathol. 2013;37:1433–1440. doi: 10.1097/PAS.0b013e31828d387b. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

