Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater
- PMID: 34066308
- PMCID: PMC8124426
- DOI: 10.3390/polym13091486
Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater
Abstract
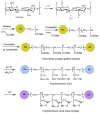
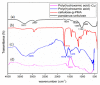
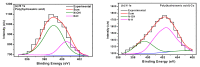
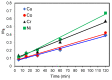
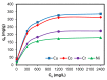
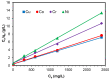
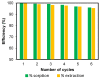
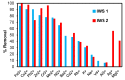
Toxic metals in the industrial wastewaters have been liable for drastic pollution hence a powerful and economical treatment technology is needed for water purification. For this reason, some pure cellulosic materials were derived from waste fiber to obtain an economical adsorbent for wastewater treatment. Conversion of cellulose into grafting materials such as poly(methyl acrylate)-grafted cellulose was performed by free radical grafting process. Consequently, poly(hydroxamic acid) ligand was produced from the grafted cellulose. The intermediate products and poly(hydroxamic acid) ligand were analyzed by FT-IR, FE-SEM, TEM, EDX, and XPS spectroscopy. The adsorption capacity (qe) of some toxic metals ions by the polymer ligand was found to be excellent, e.g., copper capacity (qe) was 346.7 mg·g-1 at pH 6. On the other hand, several metal ions such as cobalt chromium and nickel also demonstrated noteworthy sorption capacity at pH 6. The adsorption mechanism obeyed the pseudo second-order rate kinetic model due to the satisfactory correlated experimental sorption values (qe). Langmuir model isotherm study showed the significant correlation coefficient with all metal ions (R2 > 0.99), indicating that the single or monolayer adsorption was the dominant mode on the surface of the adsorbent. This polymer ligand showed good properties on reusability. The result shows that the adsorbent may be recycled for 6 cycles without any dropping of starting sorption capabilities. This polymeric ligand showed outstanding toxic metals removal magnitude, up to 90-99% of toxic metal ions can be removed from industrial wastewater.
Keywords: adsorption; heavy metals; poly(hydroxamic acid); waste fiber; wastewater.
Conflict of interest statement
The author declared no conflicts of interest.
Figures









References
-
- Ajibade F.O., Adelodun B., Lasisi K.H., Fadare O.O., Ajibade T.F., Nwogwu N.A., Sulaymon I.D., Ugya A.Y., Wang H.C., Wang A. Microbe Mediated Remediation of Environmental Contaminants. Elsevier; Amsterdam, The Netherlands: 2017. Environmental pollution and their socioeconomic impacts. - DOI
-
- Gunatilake S.K. Methods of Removing Toxic Metals for Industrial Wastewater. J. Multi. Eng. Sci. Stud. 2015;1:12–18.
-
- Rahman M.L., Sarkar S.M., Farida E.M., Arshad S.E., Sarjadi M.S., Wid N. Synthesis of tapioca cellulose-based poly(amidoxime) ligand for removal of heavy metal ions. J. Macromol. Sci. Part B Phys. 2018;57:83–99. doi: 10.1080/00222348.2018.1432179. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

