Peste des Petits Ruminants Virus Infection at the Wildlife-Livestock Interface in the Greater Serengeti Ecosystem, 2015-2019
- PMID: 34066336
- PMCID: PMC8148116
- DOI: 10.3390/v13050838
Peste des Petits Ruminants Virus Infection at the Wildlife-Livestock Interface in the Greater Serengeti Ecosystem, 2015-2019
Abstract
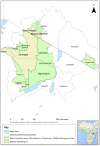
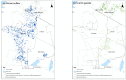
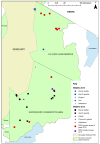
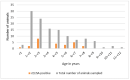
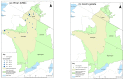
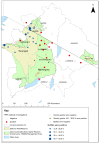
Peste des petits ruminants (PPR) is a viral disease of goats and sheep that occurs in Africa, the Middle East and Asia with a severe impact on livelihoods and livestock trade. Many wild artiodactyls are susceptible to PPR virus (PPRV) infection, and some outbreaks have threatened endangered wild populations. The role of wild species in PPRV epidemiology is unclear, which is a knowledge gap for the Global Strategy for the Control and Eradication of PPR. These studies aimed to investigate PPRV infection in wild artiodactyls in the Greater Serengeti and Amboseli ecosystems of Kenya and Tanzania. Out of 132 animals purposively sampled in 2015-2016, 19.7% were PPRV seropositive by ID Screen PPR competition enzyme-linked immunosorbent assay (cELISA; IDvet, France) from the following species: African buffalo, wildebeest, topi, kongoni, Grant's gazelle, impala, Thomson's gazelle, warthog and gerenuk, while waterbuck and lesser kudu were seronegative. In 2018-2019, a cross-sectional survey of randomly selected African buffalo and Grant's gazelle herds was conducted. The weighted estimate of PPRV seroprevalence was 12.0% out of 191 African buffalo and 1.1% out of 139 Grant's gazelles. All ocular and nasal swabs and faeces were negative by PPRV real-time reverse transcription-polymerase chain reaction (RT-qPCR). Investigations of a PPR-like disease in sheep and goats confirmed PPRV circulation in the area by rapid detection test and/or RT-qPCR. These results demonstrated serological evidence of PPRV infection in wild artiodactyl species at the wildlife-livestock interface in this ecosystem where PPRV is endemic in domestic small ruminants. Exposure to PPRV could be via spillover from infected small ruminants or from transmission between wild animals, while the relatively low seroprevalence suggests that sustained transmission is unlikely. Further studies of other major wild artiodactyls in this ecosystem are required, such as impala, Thomson's gazelle and wildebeest.
Keywords: Kenya; PPR; Tanzania; epidemiology; eradication; goats; sheep; surveillance; transboundary animal disease; wild animals.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of FAO.
Figures



References
-
- de Haan N., Kimani T., Rushton J., Lubroth J. Chapter 12 Why is small ruminant health important—peste des petits ruminants and its impact on poverty and economics? In: Munir M., editor. Peste des Petits Ruminants Virus. Springer; Berlin/Heidelberg, Germany: GmbH & Co.; Berlin, Germany: 2015. pp. 195–226.
-
- OIE-FAO . Global Strategy for the Control and Eradication of PPR World Organisation for Animal Health. United Nations Food and Agriculture Organisation; Rome, Italy: 2015. p. 255.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

