Multifaceted Role of AMPK in Viral Infections
- PMID: 34066434
- PMCID: PMC8148118
- DOI: 10.3390/cells10051118
Multifaceted Role of AMPK in Viral Infections
Abstract
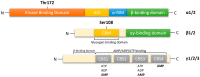
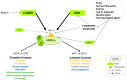
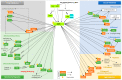
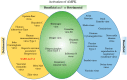
Viral pathogens often exploit host cell regulatory and signaling pathways to ensure an optimal environment for growth and survival. Several studies have suggested that 5'-adenosine monophosphate-activated protein kinase (AMPK), an intracellular serine/threonine kinase, plays a significant role in the modulation of infection. Traditionally, AMPK is a key energy regulator of cell growth and proliferation, host autophagy, stress responses, metabolic reprogramming, mitochondrial homeostasis, fatty acid β-oxidation and host immune function. In this review, we highlight the modulation of host AMPK by various viruses under physiological conditions. These intracellular pathogens trigger metabolic changes altering AMPK signaling activity that then facilitates or inhibits viral replication. Considering the COVID-19 pandemic, understanding the regulation of AMPK signaling following infection can shed light on the development of more effective therapeutic strategies against viral infectious diseases.
Keywords: AMPK; COVID-19; anabolic processes; apoptosis; autophagy; catabolic process; fatty acid metabolism; lipid metabolism; mitochondrial homeostasis; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Oakhill J.S., Chen Z.-P., Scott J.W., Steel R., Castelli L.A., Ling N., Macaulay S.L., Kemp B.E. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc. Natl. Acad. Sci. USA. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. - DOI - PMC - PubMed
-
- Mankouri J., Tedbury P.R., Gretton S., Hughes M.E., Griffin S.D.C., Dallas M.L., Green K.A., Hardie D.G., Peers C., Harris M. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA. 2010;107:11549–11554. doi: 10.1073/pnas.0912426107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

