Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease
- PMID: 34066443
- PMCID: PMC8148110
- DOI: 10.3390/vaccines9050466
Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease
Abstract
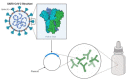
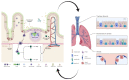
Severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) infection, the causative agent of COVID-19, now represents the sixth Public Health Emergency of International Concern (PHEIC)-as declared by the World Health Organization (WHO) since 2009. Considering that SARS-CoV-2 is mainly transmitted via the mucosal route, a therapy administered by this same route may represent a desirable approach to fight SARS-CoV-2 infection. It is now widely accepted that genetically modified microorganisms, including probiotics, represent attractive vehicles for oral or nasal mucosal delivery of therapeutic molecules. Previous studies have shown that the mucosal administration of therapeutic molecules is able to induce an immune response mediated by specific serum IgG and mucosal IgA antibodies along with mucosal cell-mediated immune responses, which effectively concur to neutralize and eradicate infections. Therefore, advances in the modulation of mucosal immune responses, and in particular the use of probiotics as live delivery vectors, may encourage prospective studies to assess the effectiveness of genetically modified probiotics for SARS-CoV-2 infection. Emerging trends in the ever-progressing field of vaccine development re-emphasize the contribution of adjuvants, along with optimization of codon usage (when designing a synthetic gene), expression level, and inoculation dose to elicit specific and potent protective immune responses. In this review, we will highlight the existing pre-clinical and clinical information on the use of genetically modified microorganisms in control strategies against respiratory and non-respiratory viruses. In addition, we will discuss some controversial aspects of the use of genetically modified probiotics in modulating the cross-talk between mucosal delivery of therapeutics and immune system modulation.
Keywords: COVID-19; SARS-CoV-2; coronavirus; mucosal immunization; probiotics; vaccines.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Chen L., Liu W., Zhang Q., Xu K., Ye G., Wu W., Sun Z., Liu F., Wu K., Zhong B., et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microbes Infect. 2020;9:313–319. doi: 10.1080/22221751.2020.1725399. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

