Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization
- PMID: 34066456
- PMCID: PMC8124704
- DOI: 10.3390/molecules26092728
Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization
Abstract
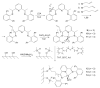
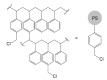
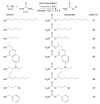
Noble metal catalysts currently dominate the landscape of chemical synthesis, but cheaper and less toxic derivatives are recently emerging as more sustainable solutions. Iron is among the possible alternative metals due to its biocompatibility and exceptional versatility. Nowadays, iron catalysts work essentially in homogeneous conditions, while heterogeneous catalysts would be better performing and more desirable systems for a broad industrial application. In this review, approaches for heterogenization of iron catalysts reported in the literature within the last two decades are summarized, and utility and critical points are discussed. The immobilization on silica of bis(arylimine)pyridyl iron complexes, good catalysts in the polymerization of olefins, is the first useful heterogeneous strategy described. Microporous molecular sieves also proved to be good iron catalyst carriers, able to provide confined geometries where olefin polymerization can occur. Same immobilizing supports (e.g., MCM-41 and MCM-48) are suitable for anchoring iron-based catalysts for styrene, cyclohexene and cyclohexane oxidation. Another excellent example is the anchoring to a Merrifield resin of an FeII-anthranilic acid complex, active in the catalytic reaction of urea with alcohols and amines for the synthesis of carbamates and N-substituted ureas, respectively. A SILP (Supported Ionic Liquid Phase) catalytic system has been successfully employed for the heterogenization of a chemoselective iron catalyst active in aldehyde hydrogenation. Finally, FeIII ions supported on polyvinylpyridine grafted chitosan made a useful heterogeneous catalytic system for C-H bond activation.
Keywords: MCM-41; SILP; alkene/alkane oxidation; carbamate synthesis; chitosan; hydrogen transfer; immobilization; ionic liquid (IL); iron catalysts; olefin polymerization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

















References
-
- Bystrzanowska M., Petkov P., Tobiszewski M. Ranking of Heterogeneous Catalysts Metals by Their Greenness. ACS Sustain. Chem. Eng. 2019;7:18434–18443. doi: 10.1021/acssuschemeng.9b04230. - DOI
-
- Chen J., Browne W.R. Photochemistry of Iron Complexes. Coord. Chem. Rev. 2018;374:15–35. doi: 10.1016/j.ccr.2018.06.008. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

