Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Human Corneal Endothelial Cells from Endoplasmic Reticulum Stress-Mediated Apoptosis
- PMID: 34066474
- PMCID: PMC8125791
- DOI: 10.3390/ijms22094930
Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Human Corneal Endothelial Cells from Endoplasmic Reticulum Stress-Mediated Apoptosis
Abstract
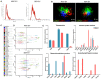
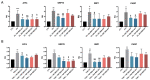
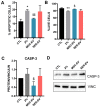
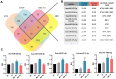
Corneal endothelial dystrophy is a relevant cause of vision loss and corneal transplantation worldwide. In the present study, we analyzed the effect of mesenchymal stem cell (MSC)-derived extracellular vesicles (MSC-EVs) in an in vitro model of corneal dystrophy, characterized by endoplasmic reticulum stress. The effects of MSC-EVs were compared with those of serum-derived EVs, reported to display a pro-angiogenic activity. MSC-EVs were able to induce a significant down-regulation of the large majority of endoplasmic reticulum stress-related genes in human corneal endothelial cells after exposure to serum deprivation and tunicamycin. In parallel, they upregulated the Akt pathway and limited caspase-3 activation and apoptosis. At variance, the effect of the serum EVs was mainly limited to Akt phosphorylation, with minimal or absent effects on endoplasmic reticulum stress modulation and apoptosis prevention. The effects of MSC-EVs were correlated to the transfer of numerous endoplasmic reticulum (ER)-stress targeting miRNAs to corneal endothelial cells. These data suggest a potential therapeutic effect of MSC-EVs for corneal endothelial endoplasmic reticulum stress, a major player in corneal endothelial dystrophy.
Keywords: corneal dystrophy; corneal endothelium; exosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Okumura N., Kitahara M., Okuda H., Hashimoto K., Ueda E., Nakahara M., Kinoshita S., Young R.D., Quantock A.J., Tourtas T., et al. Sustained activation of the unfolded protein response induces cell death in Fuchs’ endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2017;58:3697–3707. doi: 10.1167/iovs.16-21023. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

