Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
- PMID: 34066475
- PMCID: PMC8148145
- DOI: 10.3390/vaccines9050467
Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
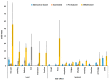
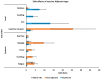
The current study systematically reviewed, summarized and meta-analyzed the clinical features of the vaccines in clinical trials to provide a better estimate of their efficacy, side effects and immunogenicity. All relevant publications were systematically searched and collected from major databases up to 12 March 2021. A total of 25 RCTs (123 datasets), 58,889 cases that received the COVID-19 vaccine and 46,638 controls who received placebo were included in the meta-analysis. In total, mRNA-based and adenovirus-vectored COVID-19 vaccines had 94.6% (95% CI 0.936-0.954) and 80.2% (95% CI 0.56-0.93) efficacy in phase II/III RCTs, respectively. Efficacy of the adenovirus-vectored vaccine after the first (97.6%; 95% CI 0.939-0.997) and second (98.2%; 95% CI 0.980-0.984) doses was the highest against receptor-binding domain (RBD) antigen after 3 weeks of injections. The mRNA-based vaccines had the highest level of side effects reported except for diarrhea and arthralgia. Aluminum-adjuvanted vaccines had the lowest systemic and local side effects between vaccines' adjuvant or without adjuvant, except for injection site redness. The adenovirus-vectored and mRNA-based vaccines for COVID-19 showed the highest efficacy after first and second doses, respectively. The mRNA-based vaccines had higher side effects. Remarkably few experienced extreme adverse effects and all stimulated robust immune responses.
Keywords: COVID-19; SARS-CoV-2; efficacy; meta-analysis; randomized clinical trial; side effect; vaccines.
Conflict of interest statement
The authors declare no competing interest. The author(s) declare no competing financial interests.
Figures



References
-
- World Health Organization . Estimating Mortality from COVID-19: Scientific Brief, 4 August 2020. World Health Organization; Genova, Switzerland: 2020.
-
- World Health Organization . Draft Landscape and Tracker of COVID-19 Candidate Vaccines. World Health Organization; Genova, Switzerland: 2021.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

