Molecular and Hormonal Mechanisms Regulating Fleshy Fruit Ripening
- PMID: 34066675
- PMCID: PMC8151651
- DOI: 10.3390/cells10051136
Molecular and Hormonal Mechanisms Regulating Fleshy Fruit Ripening
Abstract
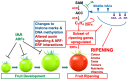
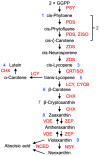
This article focuses on the molecular and hormonal mechanisms underlying the control of fleshy fruit ripening and quality. Recent research on tomato shows that ethylene, acting through transcription factors, is responsible for the initiation of tomato ripening. Several other hormones, including abscisic acid (ABA), jasmonic acid (JA) and brassinosteroids (BR), promote ripening by upregulating ethylene biosynthesis genes in different fruits. Changes to histone marks and DNA methylation are associated with the activation of ripening genes and are necessary for ripening initiation. Light, detected by different photoreceptors and operating through ELONGATED HYPOCOTYL 5(HY5), also modulates ripening. Re-evaluation of the roles of 'master regulators' indicates that MADS-RIN, NAC-NOR, Nor-like1 and other MADS and NAC genes, together with ethylene, promote the full expression of genes required for further ethylene synthesis and change in colour, flavour, texture and progression of ripening. Several different types of non-coding RNAs are involved in regulating expression of ripening genes, but further clarification of their diverse mechanisms of action is required. We discuss a model that integrates the main hormonal and genetic regulatory interactions governing the ripening of tomato fruit and consider variations in ripening regulatory circuits that operate in other fruits.
Keywords: colour; epigenetics; ethylene; flavour; fruit ripening; plant hormone; softening; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The interplay between ABA/ethylene and NAC TFs in tomato fruit ripening: a review.Plant Mol Biol. 2021 Jun;106(3):223-238. doi: 10.1007/s11103-021-01128-w. Epub 2021 Feb 25. Plant Mol Biol. 2021. PMID: 33634368 Review.
-
SlAREB1 transcriptional activation of NOR is involved in abscisic acid-modulated ethylene biosynthesis during tomato fruit ripening.Plant Sci. 2018 Nov;276:239-249. doi: 10.1016/j.plantsci.2018.07.015. Epub 2018 Jul 30. Plant Sci. 2018. PMID: 30348324
-
A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation.Plant Cell Physiol. 2014 Jan;55(1):119-35. doi: 10.1093/pcp/pct162. Epub 2013 Nov 20. Plant Cell Physiol. 2014. PMID: 24265273
-
A tomato NAC transcription factor, SlNAM1, positively regulates ethylene biosynthesis and the onset of tomato fruit ripening.Plant J. 2021 Dec;108(5):1317-1331. doi: 10.1111/tpj.15512. Epub 2021 Nov 2. Plant J. 2021. PMID: 34580960
-
Variations on a theme in fruit development: the PLE lineage of MADS-box genes in tomato (TAGL1) and other species.Planta. 2017 Aug;246(2):313-321. doi: 10.1007/s00425-017-2725-5. Epub 2017 Jun 28. Planta. 2017. PMID: 28660293 Review.
Cited by
-
In vivo and ex vivo study on cell wall components as part of the network in tomato fruit during the ripening process.Hortic Res. 2024 May 24;11(7):uhae145. doi: 10.1093/hr/uhae145. eCollection 2024 Jul. Hortic Res. 2024. PMID: 38988613 Free PMC article.
-
Global ubiquitinome analysis reveals the role of E3 ubiquitin ligase FaBRIZ in strawberry fruit ripening.J Exp Bot. 2023 Jan 1;74(1):214-232. doi: 10.1093/jxb/erac400. J Exp Bot. 2023. PMID: 36215033 Free PMC article.
-
SnRK2 subfamily I protein kinases regulate ethylene biosynthesis by phosphorylating HB transcription factors to induce ACO1 expression in apple.New Phytol. 2022 May;234(4):1262-1277. doi: 10.1111/nph.18040. Epub 2022 Mar 22. New Phytol. 2022. PMID: 35182082 Free PMC article.
-
Genome-wide characterization of the tomato GASA family identifies SlGASA1 as a repressor of fruit ripening.Hortic Res. 2022 Sep 28;10(1):uhac222. doi: 10.1093/hr/uhac222. eCollection 2023. Hortic Res. 2022. PMID: 36643743 Free PMC article.
-
Genome-wide identification and expression profile of YABBY genes in Averrhoa carambola.PeerJ. 2022 Jan 4;9:e12558. doi: 10.7717/peerj.12558. eCollection 2022. PeerJ. 2022. PMID: 35036123 Free PMC article.
References
-
- Camara B. In: Biochemistry of Fruit Ripening. Seymour G.B., Taylor J.E., Tucker G.A., editors. Chapman & Hall; London, UK: 1993. 454p
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

