Does Genetic Predisposition Contribute to the Exacerbation of COVID-19 Symptoms in Individuals with Comorbidities and Explain the Huge Mortality Disparity between the East and the West?
- PMID: 34066804
- PMCID: PMC8125927
- DOI: 10.3390/ijms22095000
Does Genetic Predisposition Contribute to the Exacerbation of COVID-19 Symptoms in Individuals with Comorbidities and Explain the Huge Mortality Disparity between the East and the West?
Abstract
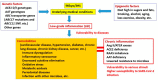
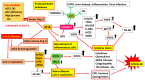
The elderly and patients with several comorbidities experience more severe cases of coronavirus disease 2019 (COVID-19) than healthy patients without underlying medical conditions. However, it is unclear why these people are prone to developing alveolar pneumonia, rapid exacerbations, and death. Therefore, we hypothesized that people with comorbidities may have a genetic predisposition that makes them more vulnerable to various factors; for example, they are likely to become more severely ill when infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To test this hypothesis, we searched the literature extensively. Polymorphisms of genes, such as those that encode angiotensin-converting enzyme 1 (ACE1), have been associated with numerous comorbidities, such as cardiovascular disease, hypertension, diabetes, chronic kidney disease, and obesity, and there are potential mechanisms to explain these associations (e.g., DD-type carriers have greater ACE1 activity, and patients with a genetic alpha-1 anti-trypsin (AAT) deficiency lack control over inflammatory mediators). Since comorbidities are associated with chronic inflammation and are closely related to the renin-angiotensin-aldosterone system (RAAS), these individuals may already have a mild ACE1/ACE2 imbalance before viral infection, which increases their risk for developing severe cases of COVID-19. However, there is still much debate about the association between ACE1 D/I polymorphism and comorbidities. The best explanation for this discrepancy could be that the D allele and DD subtypes are associated with comorbidities, but the DD genotype alone does not have an exceptionally large effect. This is also expected since the ACE1 D/I polymorphism is only an intron marker. We also discuss how polymorphisms of AAT and other genes are involved in comorbidities and the severity of SARS-CoV-2 infection. Presumably, a combination of multiple genes and non-genetic factors is involved in the establishment of comorbidities and aggravation of COVID-19.
Keywords: AAT deficiency; ACE1 DD genotype; ACE2; ADAM17; Ang II; COVID-19; RAAS; SARS-CoV-2; aggravation; comorbidities; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Impact of I/D polymorphism of angiotensin-converting enzyme 1 (ACE1) gene on the severity of COVID-19 patients.Infect Genet Evol. 2021 Jul;91:104801. doi: 10.1016/j.meegid.2021.104801. Epub 2021 Mar 4. Infect Genet Evol. 2021. PMID: 33676010 Free PMC article.
-
Angiotensin-Converting Enzyme (ACE) 1 Gene Polymorphism and Phenotypic Expression of COVID-19 Symptoms.Genes (Basel). 2021 Oct 1;12(10):1572. doi: 10.3390/genes12101572. Genes (Basel). 2021. PMID: 34680966 Free PMC article. Review.
-
[Polymorphism of RAAS genes in patients with COVID-19: comparison with frequency in population and relationship with severity of course].Ter Arkh. 2024 Oct 10;96(9):872-878. doi: 10.26442/00403660.2024.09.202849. Ter Arkh. 2024. PMID: 39467241 Russian.
-
The impact of ACE2 polymorphisms (rs1978124, rs2285666, and rs2074192) and ACE1 rs1799752 in the mortality rate of COVID-19 in different SARS-CoV-2 variants.Hum Genomics. 2023 Jun 16;17(1):54. doi: 10.1186/s40246-023-00501-8. Hum Genomics. 2023. PMID: 37328914 Free PMC article.
-
Single Nucleotide Variants (SNVs) of Angiotensin-Converting Enzymes (ACE1 and ACE2): A Plausible Explanation for the Global Variation in COVID-19 Prevalence.J Renin Angiotensin Aldosterone Syst. 2023 Apr 3;2023:9668008. doi: 10.1155/2023/9668008. eCollection 2023. J Renin Angiotensin Aldosterone Syst. 2023. PMID: 37051471 Free PMC article. Review.
Cited by
-
Comparison of DASH diet score and total antioxidant capacity of diet on serum levels of TMPRSS-2, inflammatory biomarkers, and disease severity in COVID-19 patients: A case-control study.Food Sci Nutr. 2024 Feb 14;12(5):3552-3562. doi: 10.1002/fsn3.4024. eCollection 2024 May. Food Sci Nutr. 2024. PMID: 38726461 Free PMC article.
-
Prediction models of COVID-19 fatality in nine Peruvian provinces: A secondary analysis of the national epidemiological surveillance system.PLOS Glob Public Health. 2024 Jan 29;4(1):e0002854. doi: 10.1371/journal.pgph.0002854. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 38285714 Free PMC article.
-
Vitamin D Deficiency and COVID-19: A Biological Database Study on Pathways and Gene-Disease Associations.Int J Mol Sci. 2022 Nov 17;23(22):14256. doi: 10.3390/ijms232214256. Int J Mol Sci. 2022. PMID: 36430729 Free PMC article.
-
Genetic variants in TMPRSS2 influence SARS-CoV-2 infection susceptibility within Mexican Mestizos.Front Genet. 2025 Apr 14;16:1558189. doi: 10.3389/fgene.2025.1558189. eCollection 2025. Front Genet. 2025. PMID: 40296872 Free PMC article.
-
Homozygous-Recessive Characteristics as a Biomarker of Predisposition for COVID-19.Clin Nurs Res. 2023 Mar;32(3):589-600. doi: 10.1177/10547738221147754. Epub 2023 Jan 25. Clin Nurs Res. 2023. PMID: 36695163 Free PMC article.
References
-
- Margallo N.L., Diaz M., Lim P.P. 2019 Novel Coronavirus Pandemic: What Do We Know? S D Med. 2020;73:262–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

