Identification and Functional Annotation of Genes Related to Bone Stability in Laying Hens Using Random Forests
- PMID: 34066823
- PMCID: PMC8151682
- DOI: 10.3390/genes12050702
Identification and Functional Annotation of Genes Related to Bone Stability in Laying Hens Using Random Forests
Abstract
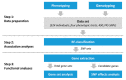
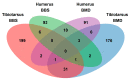
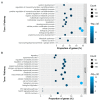
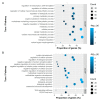
Skeletal disorders, including fractures and osteoporosis, in laying hens cause major welfare and economic problems. Although genetics have been shown to play a key role in bone integrity, little is yet known about the underlying genetic architecture of the traits. This study aimed to identify genes associated with bone breaking strength and bone mineral density of the tibiotarsus and the humerus in laying hens. Potentially informative single nucleotide polymorphisms (SNP) were identified using Random Forests classification. We then searched for genes known to be related to bone stability in close proximity to the SNPs and identified 16 potential candidates. Some of them had human orthologues. Based on our findings, we can support the assumption that multiple genes determine bone strength, with each of them having a rather small effect, as illustrated by our SNP effect estimates. Furthermore, the enrichment analysis showed that some of these candidates are involved in metabolic pathways critical for bone integrity. In conclusion, the identified candidates represent genes that may play a role in the bone integrity of chickens. Although further studies are needed to determine causality, the genes reported here are promising in terms of alleviating bone disorders in laying hens.
Keywords: Random Forests; bone breaking strength; bone mineral density; gene set enrichment analysis; osteoporosis; single nucleotide polymorphism; skeletal integrity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genome-wide association study for bone strength in laying hens.J Anim Sci. 2018 Jun 29;96(7):2525-2535. doi: 10.1093/jas/sky157. J Anim Sci. 2018. PMID: 29701819 Free PMC article.
-
Novel Polymorphisms in RAPGEF6 Gene Associated with Egg-Laying Rate in Chinese Jing Hong Chicken using Genome-Wide SNP Scan.Genes (Basel). 2019 May 20;10(5):384. doi: 10.3390/genes10050384. Genes (Basel). 2019. PMID: 31137587 Free PMC article.
-
Comparison of bone volume and strength as measures of skeletal integrity in caged laying hens with access to perches.Res Vet Sci. 1993 Mar;54(2):202-6. doi: 10.1016/0034-5288(93)90057-m. Res Vet Sci. 1993. PMID: 8460260
-
Genetics of osteoporosis.Biochem Biophys Res Commun. 2014 Sep 19;452(2):287-93. doi: 10.1016/j.bbrc.2014.07.141. Epub 2014 Aug 16. Biochem Biophys Res Commun. 2014. PMID: 25139232 Review.
-
Welfare implications of avian osteoporosis.Poult Sci. 2004 Feb;83(2):184-92. doi: 10.1093/ps/83.2.184. Poult Sci. 2004. PMID: 14979568 Review.
Cited by
-
Study of genes polymorphisms in RANK/RANKL/OPG and WNT signaling pathways and their associations with bone parameters in broiler chicken.Heliyon. 2023 Nov 11;9(11):e22371. doi: 10.1016/j.heliyon.2023.e22371. eCollection 2023 Nov. Heliyon. 2023. PMID: 38053912 Free PMC article.
-
Identification of the genetic basis of sow pelvic organ prolapse.Front Genet. 2023 Apr 18;14:1154713. doi: 10.3389/fgene.2023.1154713. eCollection 2023. Front Genet. 2023. PMID: 37144137 Free PMC article.
-
Deciphering Pleiotropic Signatures of Regulatory SNPs in Zea mays L. Using Multi-Omics Data and Machine Learning Algorithms.Int J Mol Sci. 2022 May 4;23(9):5121. doi: 10.3390/ijms23095121. Int J Mol Sci. 2022. PMID: 35563516 Free PMC article.
-
Special Issue: Poultry Genetics, Breeding and Biotechnology.Genes (Basel). 2021 Oct 29;12(11):1744. doi: 10.3390/genes12111744. Genes (Basel). 2021. PMID: 34828350 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

