A Tryptophan-Deficient Diet Induces Gut Microbiota Dysbiosis and Increases Systemic Inflammation in Aged Mice
- PMID: 34066870
- PMCID: PMC8125914
- DOI: 10.3390/ijms22095005
A Tryptophan-Deficient Diet Induces Gut Microbiota Dysbiosis and Increases Systemic Inflammation in Aged Mice
Abstract
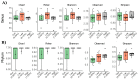
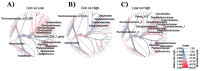
The gut microflora is a vital component of the gastrointestinal (GI) system that regulates local and systemic immunity, inflammatory response, the digestive system, and overall health. Older people commonly suffer from inadequate nutrition or poor diets, which could potentially alter the gut microbiota. The essential amino acid (AA) tryptophan (TRP) is a vital diet component that plays a critical role in physiological stress responses, neuropsychiatric health, oxidative systems, inflammatory responses, and GI health. The present study investigates the relationship between varied TRP diets, the gut microbiome, and inflammatory responses in an aged mouse model. We fed aged mice either a TRP-deficient (0.1%), TRP-recommended (0.2%), or high-TRP (1.25%) diet for eight weeks and observed changes in the gut bacterial environment and the inflammatory responses via cytokine analysis (IL-1a, IL-6, IL-17A, and IL-27). The mice on the TRP-deficient diets showed changes in their bacterial abundance of Coriobacteriia class, Acetatifactor genus, Lachnospiraceae family, Enterococcus faecalis species, Clostridium sp genus, and Oscillibacter genus. Further, these mice showed significant increases in IL-6, IL-17A, and IL-1a and decreased IL-27 levels. These data suggest a direct association between dietary TRP content, the gut microbiota microenvironment, and inflammatory responses in aged mice models.
Keywords: dysbiosis; gut; microbiota; systemic inflammation; tryptophan.
Conflict of interest statement
The authors also declare that there is no other conflict of interest regarding the publication of this manuscript.
Figures




Similar articles
-
Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice.BMC Microbiol. 2019 Aug 20;19(1):193. doi: 10.1186/s12866-019-1557-9. BMC Microbiol. 2019. PMID: 31429703 Free PMC article.
-
Early-Life Stress Modulates Gut Microbiota and Peripheral and Central Inflammation in a Sex-Dependent Manner.Int J Mol Sci. 2021 Feb 14;22(4):1899. doi: 10.3390/ijms22041899. Int J Mol Sci. 2021. PMID: 33672958 Free PMC article.
-
Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet.mBio. 2017 May 23;8(3):e00470-17. doi: 10.1128/mBio.00470-17. mBio. 2017. PMID: 28536285 Free PMC article.
-
Interactive relationship between Trp metabolites and gut microbiota: The impact on human pathology of disease.J Appl Microbiol. 2022 Jun;132(6):4186-4207. doi: 10.1111/jam.15533. Epub 2022 Mar 29. J Appl Microbiol. 2022. PMID: 35304801 Review.
-
Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health.Gut Microbes. 2020 Nov 9;12(1):1802866. doi: 10.1080/19490976.2020.1802866. Gut Microbes. 2020. PMID: 32835590 Free PMC article. Review.
Cited by
-
Tryptophan Metabolism in Alzheimer's Disease with the Involvement of Microglia and Astrocyte Crosstalk and Gut-Brain Axis.Aging Dis. 2024 Oct 1;15(5):2168-2190. doi: 10.14336/AD.2024.0134. Aging Dis. 2024. PMID: 38916729 Free PMC article. Review.
-
Association between metabolites in tryptophan-kynurenine pathway and inflammatory bowel disease: a two-sample Mendelian randomization.Sci Rep. 2024 Jan 2;14(1):201. doi: 10.1038/s41598-023-50990-9. Sci Rep. 2024. PMID: 38167867 Free PMC article.
-
Multiomics Study Reveals Enterococcus and Subdoligranulum Are Beneficial to Necrotizing Enterocolitis.Front Microbiol. 2021 Nov 18;12:752102. doi: 10.3389/fmicb.2021.752102. eCollection 2021. Front Microbiol. 2021. PMID: 34867873 Free PMC article.
-
Dietary oxidized beef protein alters gut microbiota and induces colonic inflammatory damage in C57BL/6 mice.Front Nutr. 2022 Sep 2;9:980204. doi: 10.3389/fnut.2022.980204. eCollection 2022. Front Nutr. 2022. PMID: 36118776 Free PMC article.
-
Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation.Blood. 2023 Apr 6;141(14):1691-1707. doi: 10.1182/blood.2022017514. Blood. 2023. PMID: 36638348 Free PMC article.
References
-
- Shlisky J., Bloom D.E., Beaudreault A.R., Tucker K.L., Keller H.H., Freund-Levi Y., Fielding R.A., Cheng F.W., Jensen G.L., Wu D., et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017;8:17–26. doi: 10.3945/an.116.013474. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

