Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events
- PMID: 34066908
- PMCID: PMC8124725
- DOI: 10.3390/cancers13092237
Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events
Abstract
Polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose (poly-ICLC) is a synthetic double-stranded viral RNA analog widely tested as a component of human therapeutic cancer vaccines and as a standalone agent for treating human cancers. However, there are no reports on the use of poly-ICLC for treating canine cancers. This study aimed to investigate the clinical efficacy, quality of life (QL), and adverse events of poly-ICLC treatment in dogs with advanced cancers. The treatment protocol consisted of weekly intratumoral doses of poly-ICLC. The canine patients underwent clinical, laboratory, and imaging tests, and their owners answered weekly QL questionnaires. Fourteen canine patients with different types of spontaneous advanced tumors were enrolled. Most dogs had received prior conventional therapies. Five dogs received at least 12 doses of poly-ICLC: the injected tumor was stable in three dogs, there was a partial response in one, and the injected tumor significantly enlarged in the other. The QL scoring remained stable or increased in most cases. Mild adverse events related to poly-ICLC were observed in 10 of the 14 patients. The data showed that intratumoral poly-ICLC therapy was well tolerated in dogs with advanced cancers, with clinical benefit and improved QL scores observed in some dogs.
Keywords: cancer; dog; quality of life; therapy; viral RNA.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, but approved the decision to publish the results.
Figures
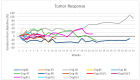

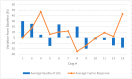
References
-
- Caskey M., Lefebvre F., Filali-Mouhim A., Cameron M.J., Goulet J.P., Haddad E.K., Breton G., Trumpfheller C., Pollak S., Shimeliovich I., et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 2011;208:2357–2366. doi: 10.1084/jem.20111171. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

