Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design
- PMID: 34066921
- PMCID: PMC8148523
- DOI: 10.3390/pharmaceutics13050668
Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design
Abstract
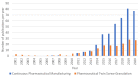
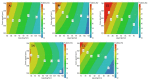
Continuous twin screw wet granulation is one of the key continuous manufacturing technologies that have gained significant interest in the pharmaceutical industry as well as in academia over the last ten years. Given its considerable advantages compared to wet granulation techniques operated in batch mode such as high shear granulation and fluid bed granulation, several equipment manufacturers have designed their own manufacturing setup. This has led to a steep increase in the research output in this field. However, most studies still focused on a single (often placebo) formulation, hence making it difficult to assess the general validity of the obtained results. Therefore, current review provides an overview of recent progress in the field of continuous twin screw wet granulation, with special focus on the importance of the formulation aspect and raw material properties. It gives practical guidance for novel and more experienced users of this technique and highlights some of the unmet needs that require further research.
Keywords: continuous manufacturing; formulation development; innovation; process development; review; twin screw granulation; wet granulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- U.S. Food and Drug Administration . Quality Considerations for Continuous Manufacturing—Guidance for Industry—Draft Guidance. U.S. Food and Drug Administration; Silver Spring, MD, USA: 2019.
-
- Portier C., Pandelaere K., Delaet U., Vigh T., Kumar A., Di Pretoro G., De Beer T., Vervaet C., Vanhoorne V. Continuous twin screw granulation: Influence of process and formulation variables on granule quality attributes of model formulations. Int. J. Pharm. 2020;576:118981. doi: 10.1016/j.ijpharm.2019.118981. - DOI - PubMed
-
- Lee S.L., O’Connor T.F., Yang X., Cruz C.N., Chatterjee S., Madurawe R.D., Moore C.M.V., Yu L.X., Woodcock J. Modernizing Pharmaceutical Manufacturing: From Batch to Continuous Production. J. Pharm. Innov. 2015;10:191–199. doi: 10.1007/s12247-015-9215-8. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

