Molecular Mechanisms Associated with ROS-Dependent Angiogenesis in Lower Extremity Artery Disease
- PMID: 34066926
- PMCID: PMC8148529
- DOI: 10.3390/antiox10050735
Molecular Mechanisms Associated with ROS-Dependent Angiogenesis in Lower Extremity Artery Disease
Abstract
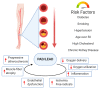
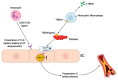
Currently, atherosclerosis, which affects the vascular bed of all vital organs and tissues, is considered as a leading cause of death. Most commonly, atherosclerosis involves coronary and peripheral arteries, which results in acute (e.g., myocardial infarction, lower extremities ischemia) or chronic (persistent ischemia leading to severe heart failure) consequences. All of them have a marked unfavorable impact on the quality of life and are associated with increased mortality and morbidity in human populations. Lower extremity artery disease (LEAD, also defined as peripheral artery disease, PAD) refers to atherosclerotic occlusive disease of the lower extremities, where partial or complete obstruction of peripheral arteries is observed. Decreased perfusion can result in ischemic pain, non-healing wounds, and ischemic ulcers, and significantly reduce the quality of life. However, the progressive atherosclerotic changes cause stimulation of tissue response processes, like vessel wall remodeling and neovascularization. These mechanisms of adapting the vascular network to pathological conditions seem to play a key role in reducing the impact of the changes limiting the flow of blood. Neovascularization as a response to ischemia induces sprouting and expansion of the endothelium to repair and grow the vessels of the circulatory system. Neovascularization consists of three different biological processes: vasculogenesis, angiogenesis, and arteriogenesis. Both molecular and environmental factors that may affect the process of development and growth of blood vessels were analyzed. Particular attention was paid to the changes taking place during LEAD. It is important to consider the molecular mechanisms underpinning vessel growth. These mechanisms will also be examined in the context of diseases commonly affecting blood vessel function, or those treatable in part by manipulation of angiogenesis. Furthermore, it may be possible to induce the process of blood vessel development and growth to treat peripheral vascular disease and wound healing. Reactive oxygen species (ROS) play an important role in regulation of essential cellular signaling pathways such as cell differentiation, proliferation, migration and apoptosis. With regard to the repair processes taking place during diseases such as LEAD, prospective therapeutic methods have been described that could significantly improve the treatment of vessel diseases in the future. Summarizing, regenerative medicine holds the potential to transform the therapeutic methods in heart and vessel diseases treatment.
Keywords: angiogenesis; atherosclerosis; neovascularization; peripheral arterial diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rizzi A., Benagiano V., Ribatti D. Angiogenesis versus arteriogenesis. Rom. J. Morphol. Embryol. 2017;58:15–19. - PubMed
-
- Fowkes F.G.R., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., Norman P.E., Sampson U.K.A., Williams L.J., Mensah G.A., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

