Epigenetic Regulation of Neuroinflammation in Parkinson's Disease
- PMID: 34066949
- PMCID: PMC8125491
- DOI: 10.3390/ijms22094956
Epigenetic Regulation of Neuroinflammation in Parkinson's Disease
Abstract
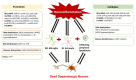
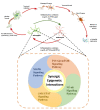
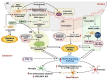
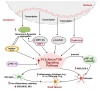
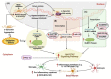
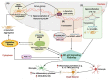
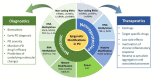
Neuroinflammation is one of the most significant factors involved in the initiation and progression of Parkinson's disease. PD is a neurodegenerative disorder with a motor disability linked with various complex and diversified risk factors. These factors trigger myriads of cellular and molecular processes, such as misfolding defective proteins, oxidative stress, mitochondrial dysfunction, and neurotoxic substances that induce selective neurodegeneration of dopamine neurons. This neuronal damage activates the neuronal immune system, including glial cells and inflammatory cytokines, to trigger neuroinflammation. The transition of acute to chronic neuroinflammation enhances the susceptibility of inflammation-induced dopaminergic neuron damage, forming a vicious cycle and prompting an individual to PD development. Epigenetic mechanisms recently have been at the forefront of the regulation of neuroinflammatory factors in PD, proposing a new dawn for breaking this vicious cycle. This review examined the core epigenetic mechanisms involved in the activation and phenotypic transformation of glial cells mediated neuroinflammation in PD. We found that epigenetic mechanisms do not work independently, despite being coordinated with each other to activate neuroinflammatory pathways. In this regard, we attempted to find the synergic correlation and contribution of these epigenetic modifications with various neuroinflammatory pathways to broaden the canvas of underlying pathological mechanisms involved in PD development. Moreover, this study highlighted the dual characteristics (neuroprotective/neurotoxic) of these epigenetic marks, which may counteract PD pathogenesis and make them potential candidates for devising future PD diagnosis and treatment.
Keywords: Parkinson’s disease; astrocytes; epigenetics; microglia; neurodegeneration; neuroinflammation.
Conflict of interest statement
The authors of this study declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

