Post-Transcriptional Regulation of Viral RNA through Epitranscriptional Modification
- PMID: 34066974
- PMCID: PMC8151693
- DOI: 10.3390/cells10051129
Post-Transcriptional Regulation of Viral RNA through Epitranscriptional Modification
Abstract
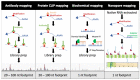
The field of mRNA modifications has been steadily growing in recent years as technologies have improved and the importance of these residues became clear. However, a subfield has also arisen, specifically focused on how these modifications affect viral RNA, with the possibility that viruses can also be used as a model to best determine the role that these modifications play on cellular mRNAs. First, virologists focused on the most abundant internal mRNA modification, m6A, mapping this modification and elucidating its effects on the RNA of a wide range of RNA and DNA viruses. Next, less common RNA modifications including m5C, Nm and ac4C were investigated and also found to be present on viral RNA. It now appears that viral RNA is littered with a multitude of RNA modifications. In biological systems that are under constant evolutionary pressure to out compete both the host as well as newly arising viral mutants, it poses an interesting question about what evolutionary benefit these modifications provide as it seems evident, at least to this author, that these modifications have been selected for. In this review, I discuss how RNA modifications are identified on viral RNA and the roles that have now been uncovered for these modifications in regard to viral replication. Finally, I propose some interesting avenues of research that may shed further light on the exact role that these modifications play in viral replication.
Keywords: 5-methylcytosine; HIV-1; N6-methyladenosine; RNA; epitranscriptomic; mapping; modification; pseudouridine; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of Viral Infection by the RNA Modification N6-Methyladenosine.Annu Rev Virol. 2019 Sep 29;6(1):235-253. doi: 10.1146/annurev-virology-092818-015559. Epub 2019 Jul 5. Annu Rev Virol. 2019. PMID: 31283446 Free PMC article. Review.
-
Epitranscriptomic regulation of viral replication.Biochim Biophys Acta Gene Regul Mech. 2017 Apr;1860(4):460-471. doi: 10.1016/j.bbagrm.2017.02.002. Epub 2017 Feb 17. Biochim Biophys Acta Gene Regul Mech. 2017. PMID: 28219769 Review.
-
[Progress in epigenetic modification of mRNA and the function of m6A modification].Sheng Wu Gong Cheng Xue Bao. 2019 May 25;35(5):775-783. doi: 10.13345/j.cjb.180416. Sheng Wu Gong Cheng Xue Bao. 2019. PMID: 31222996 Review. Chinese.
-
Viral Epitranscriptomics.J Virol. 2017 Apr 13;91(9):e02263-16. doi: 10.1128/JVI.02263-16. Print 2017 May 1. J Virol. 2017. PMID: 28250115 Free PMC article. Review.
-
Deciphering the Epitranscriptomic Signatures in Cell Fate Determination and Development.Stem Cell Rev Rep. 2019 Aug;15(4):474-496. doi: 10.1007/s12015-019-09894-3. Stem Cell Rev Rep. 2019. PMID: 31123982 Review.
Cited by
-
Nanopore-Based Detection of Viral RNA Modifications.mBio. 2022 Jun 28;13(3):e0370221. doi: 10.1128/mbio.03702-21. Epub 2022 May 17. mBio. 2022. PMID: 35579392 Free PMC article. Review.
-
Plant YTHDF proteins are direct effectors of antiviral immunity against an N6-methyladenosine-containing RNA virus.EMBO J. 2023 Sep 18;42(18):e113378. doi: 10.15252/embj.2022113378. Epub 2023 Jul 11. EMBO J. 2023. PMID: 37431920 Free PMC article.
-
From RNA World to SARS-CoV-2: The Edited Story of RNA Viral Evolution.Cells. 2021 Jun 20;10(6):1557. doi: 10.3390/cells10061557. Cells. 2021. PMID: 34202997 Free PMC article. Review.
-
The Applications of Nanopore Sequencing Technology in Animal and Human Virus Research.Viruses. 2024 May 16;16(5):798. doi: 10.3390/v16050798. Viruses. 2024. PMID: 38793679 Free PMC article. Review.
-
TWIK-related acid-sensitive K+ channel 2 promotes renal fibrosis by inducing cell-cycle arrest.iScience. 2022 Nov 17;25(12):105620. doi: 10.1016/j.isci.2022.105620. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465115 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

