Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films
- PMID: 34067109
- PMCID: PMC8124804
- DOI: 10.3390/polym13091507
Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films
Abstract
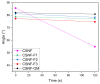
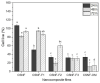
Herein, the effect of three deterpenated fractions from Origanum majorana L. essential oil on the physicochemical, mechanical and biological properties of chitosan/β-chitin nanofibers-based nanocomposite films were investigated. In general, the incorporation of Origanum majorana L. original essential oil or its deterpenated fractions increases the opacity of the nanocomposite films and gives them a yellowish color. The water solubility decreases from 58% for chitosan/β-chitin nanofibers nanocomposite film to around 32% for the nanocomposite films modified with original essential oil or its deterpenated fractions. Regarding the thermal stability, no major changes were observed, and the mechanical properties decreased. Interestingly, data show differences on the biological properties of the materials depending on the incorporated deterpenated fraction of Origanum majorana L. essential oil. The nanocomposite films prepared with the deterpenated fractions with a high concentration of oxygenated terpene derivatives show the best antifungal activity against Aspergillus niger, with fungal growth inhibition of around 85.90%. Nonetheless, the only nanocomposite film that does not present cytotoxicity on the viability of L929 fibroblast cells after 48 and 72 h is the one prepared with the fraction presenting the higher terpenic hydrocarbon content (87.92%). These results suggest that the composition of the deterpenated fraction plays an important role in determining the biological properties of the nanocomposite films.
Keywords: Origanum majorana L. essential oil; beta-chitin nanofibers; biological properties; chitosan; deterpenated fractions; nanocomposite films.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ribeiro-Santos R., Andrade M., De Melo N.R., Sanches-Silva A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017;61:132–140. doi: 10.1016/j.tifs.2016.11.021. - DOI
-
- Fernández-Marín R., Labidi J., Andrés M.Á., Fernandes S.C.M. Using α-chitin nanocrystals to improve the final properties of poly (vinyl alcohol) films with Origanum vulgare essential oil. Polym. Degrad. Stab. 2020;179 doi: 10.1016/j.polymdegradstab.2020.109227. - DOI
-
- Lago S., Rodríguez H., Soto A., Arce A. Deterpenation of citrus essential oil by liquid-liquid extraction with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ionic liquids. J. Chem. Eng. Data. 2011;56:1273–1281. doi: 10.1021/je1011339. - DOI
-
- Silvestre W.P., Agostini F., Muniz L.A.R., Pauletti G.F. Fractionating of green Mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016;178:90–94. doi: 10.1016/j.jfoodeng.2016.01.011. - DOI
LinkOut - more resources
Full Text Sources

