Production of Cyclic Anhydride-Modified Starches
- PMID: 34067113
- PMCID: PMC8125099
- DOI: 10.3390/polym13091504
Production of Cyclic Anhydride-Modified Starches
Abstract
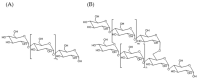
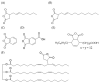
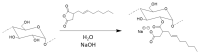
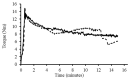
Modified starches offer a biodegradable, readily available, and cost-effective alternative to petroleum-based products. The reaction of alkenylsuccinic anhydrides (ASAs), in particular, is an efficient method to produce amphiphilic starches with numerous applications in different areas. While ASAs are typically derived from petroleum sources, maleated soybean oil can also be used in an effort to produce materials from renewable sources. The reaction of gelatinized waxy maize starch with octenylsuccinic anhydride (OSA), dodecenylsuccinic anhydride (DDSA), a maleated fatty acid (TENAX 2010), phthalic anhydride (PA), 1,2,4-benzenetricarboxylic acid anhydride (trimellitic anhydride, TMA), and three maleated soybean oil samples, was investigated under different conditions. To minimize the reaction time and the amount of water required, the outcome of the esterification reaction was compared for starch dispersions in benchtop dispersed reactions, for starch melts in a heated torque rheometer, and for reactive extrusion in a pilot plant scale twin-screw extruder. The extent of reaction was quantified by 1H NMR analysis, and changes in molecular weight and diameter were monitored by gel permeation chromatography (GPC) analysis. The outcome of the reactions varied markedly in terms of reaction efficiency (RE), molecular weight distribution, and average hydrodynamic diameter, for the products derived from the different maleated reagents used, as well as for the different reaction protocols.
Keywords: alkenylsuccinic anhydride (ASA); esterification; reactive extrusion; soybean oil; starch.
Conflict of interest statement
Julien Mesnager and Michael Kuska were employed by EcoSynthetix during the investigation. Ryan C. Amos and Mario Gauthier declare no conflict of interest.
Figures
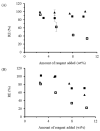
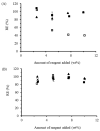
 ) and starch with water and OSA (
) and starch with water and OSA ( ).
).
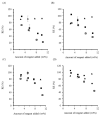

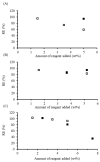
 ), and for starch, water and MSO-2.0 without base (
), and for starch, water and MSO-2.0 without base ( ) and with base (
) and with base ( ).
).
References
-
- Zhou J., Ren L., Tong J., Xie L., Liu Z. Surface esterification of corn starch films: Reaction with dodecenyl succinic anhydride. Carbohydr. Polym. 2009;78:888–893. doi: 10.1016/j.carbpol.2009.07.017. - DOI
-
- Bai Y., Shi Y.-C., Herrera A., Prakash O. Study of octenyl succinic anhydride-modified waxy maize starch by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2011;83:407–413. doi: 10.1016/j.carbpol.2010.07.053. - DOI
-
- Ma Z., Zhao S., Cheng K., Zhang X., Xu X., Zhang L. Molecular weight and chain conformation of amylopectin from rice starch. J. Appl. Polym. Sci. 2007;104:3124–3128. doi: 10.1002/app.26141. - DOI
-
- You S., Fiedorowicz M., Lim S.-T. Molecular characterization of wheat amylopectins by multiangle laser light scattering analysis. Cereal Chem. J. 1999;76:116–121. doi: 10.1094/CCHEM.1999.76.1.116. - DOI
LinkOut - more resources
Full Text Sources

