The Chemistry Behind ADCs
- PMID: 34067144
- PMCID: PMC8152005
- DOI: 10.3390/ph14050442
The Chemistry Behind ADCs
Abstract
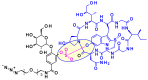
Combining the selective targeting of tumor cells through antigen-directed recognition and potent cell-killing by cytotoxic payloads, antibody-drug conjugates (ADCs) have emerged in recent years as an efficient therapeutic approach for the treatment of various cancers. Besides a number of approved drugs already on the market, there is a formidable follow-up of ADC candidates in clinical development. While selection of the appropriate antibody (A) and drug payload (D) is dictated by the pharmacology of the targeted disease, one has a broader choice of the conjugating linker (C). In the present paper, we review the chemistry of ADCs with a particular emphasis on the medicinal chemistry perspective, focusing on the chemical methods that enable the efficient assembly of the ADC from its three components and the controlled release of the drug payload.
Keywords: antibody-drug conjugates; attachment point; conjugation; linker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


































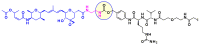
























References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

