Hyperferritinemia-A Clinical Overview
- PMID: 34067164
- PMCID: PMC8125175
- DOI: 10.3390/jcm10092008
Hyperferritinemia-A Clinical Overview
Abstract
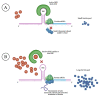
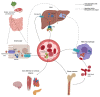
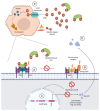
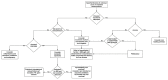
Ferritin is one of the most frequently requested laboratory tests in primary and secondary care, and levels often deviate from reference ranges. Serving as an indirect marker for total body iron stores, low ferritin is highly specific for iron deficiency. Hyperferritinemia is, however, a non-specific finding, which is frequently overlooked in general practice. In routine medical practice, only 10% of cases are related to an iron overload, whilst the rest is seen as a result of acute phase reactions and reactive increases in ferritin due to underlying conditions. Differentiation of the presence or absence of an associated iron overload upon hyperferritinemia is essential, although often proves to be complex. In this review, we have performed a review of a selection of the literature based on the authors' own experiences and assessments in accordance with international recommendations and guidelines. We address the biology, etiology, and epidemiology of hyperferritinemia. Finally, an algorithm for the diagnostic workup and management of hyperferritinemia is proposed, and general principles regarding the treatment of iron overload are discussed.
Keywords: ferritin; hemochromatosis; inflammation; iron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Beaton M.D., Adams P.C. Treatment of hyperferritinemia. Ann. Hepatol. 2012;11:294–300. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

