Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections
- PMID: 34067256
- PMCID: PMC8196896
- DOI: 10.3390/ijms22115461
Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections
Abstract
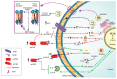
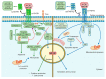
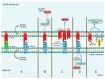
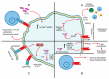
Tumor necrosis factor (TNF) is one of the main cytokines regulating a pro-inflammatory environment. It has been related to several cell functions, for instance, phagocytosis, apoptosis, proliferation, mitochondrial dynamic. Moreover, during mycobacterial infections, TNF plays an essential role to maintain granuloma formation. Several effector mechanisms have been implicated according to the interactions of the two active forms, soluble TNF (solTNF) and transmembrane TNF (tmTNF), with their receptors TNFR1 and TNFR2. We review the impact of these interactions in the context of mycobacterial infections. TNF is tightly regulated by binding to receptors, however, during mycobacterial infections, upstream activation signalling pathways may be influenced by key regulatory factors either at the membrane or cytosol level. Detailing the structure and activation pathways used by TNF and its receptors, such as its interaction with solTNF/TNFRs versus tmTNF/TNFRs, may bring a better understanding of the molecular mechanisms involved in activation pathways which can be helpful for the development of new therapies aimed at being more efficient against mycobacterial infections.
Keywords: TNF receptors; mycobacterial infections; tumor necrosis factor; tumor necrosis factor-α converting enzyme (TACE).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

