Modified Nucleosides, Nucleotides and Nucleic Acids via Click Azide-Alkyne Cycloaddition for Pharmacological Applications
- PMID: 34067312
- PMCID: PMC8196910
- DOI: 10.3390/molecules26113100
Modified Nucleosides, Nucleotides and Nucleic Acids via Click Azide-Alkyne Cycloaddition for Pharmacological Applications
Abstract
The click azide = alkyne 1,3-dipolar cycloaddition (click chemistry) has become the approach of choice for bioconjugations in medicinal chemistry, providing facile reaction conditions amenable to both small and biological molecules. Many nucleoside analogs are known for their marked impact in cancer therapy and for the treatment of virus diseases and new targeted oligonucleotides have been developed for different purposes. The click chemistry allowing the tolerated union between units with a wide diversity of functional groups represents a robust means of designing new hybrid compounds with an extraordinary diversity of applications. This review provides an overview of the most recent works related to the use of click chemistry methodology in the field of nucleosides, nucleotides and nucleic acids for pharmacological applications.
Keywords: 1,2,3-trizole; azide-alkyne cycloaddition; bioisosteres; click chemistry; nucleic acid; nucleosides; oligonucleotides; pharmaceutical approach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
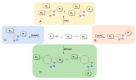
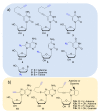

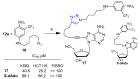
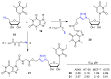
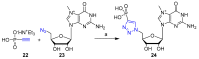
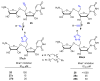
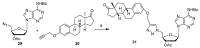
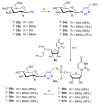




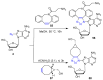



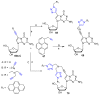
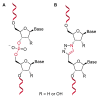
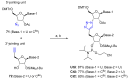
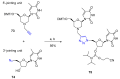
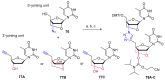
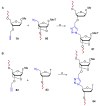
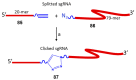

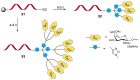
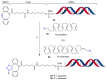
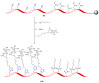
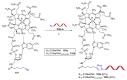
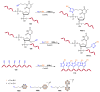
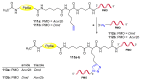
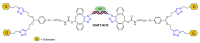
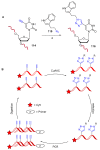
References
-
- Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. - DOI - PubMed
-
- Huisgen R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963;2:565–598. doi: 10.1002/anie.196305651. - DOI
-
- Huisgen R. 1,3-Dipolar cycloaddition chemistry. Volumes 1 and 2. Edited by Albert Padwa. John Wiley and Sons. New York, 1984. Volume 1: XIII + 817 pages. Volume 2: XIII + 704 pages. ISBN 0-471-08364-X (set). $295.00 for the two-volume set. J. Heterocycl. Chem. 1986;23:1899. doi: 10.1002/jhet.5570230658. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

