Conformational Control of DNA Origami by DNA Oligomers, Intercalators and UV Light
- PMID: 34067324
- PMCID: PMC8163164
- DOI: 10.3390/mps4020038
Conformational Control of DNA Origami by DNA Oligomers, Intercalators and UV Light
Abstract
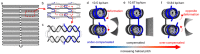
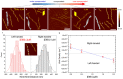
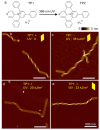
DNA origami has garnered great attention due to its excellent programmability and precision. It offers a powerful means to create complex nanostructures which may not be possible by other methods. The macromolecular structures may be used as static templates for arranging proteins and other molecules. They are also capable of undergoing structural transformation in response to external signals, which may be exploited for sensing and actuation at the nanoscale. Such on-demand reconfigurations are executed mostly by DNA oligomers through base-pairing and/or strand displacement, demonstrating drastic shape changes between two different states, for example, open and close. Recent studies have developed new mechanisms to modulate the origami conformation in a controllable, progressive manner. Here we present several methods for conformational control of DNA origami nanostructures including chemical adducts and UV light as well as widely applied DNA oligomers. The detailed methods should be useful for beginners in the field of DNA nanotechnology.
Keywords: DNA origami; UV; conformation; intercalator; strand displacement; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dynamic and Progressive Control of DNA Origami Conformation by Modulating DNA Helicity with Chemical Adducts.ACS Nano. 2016 May 24;10(5):4989-96. doi: 10.1021/acsnano.6b01339. Epub 2016 Apr 12. ACS Nano. 2016. PMID: 27057775
-
DNA-Nanotechnology-Enabled Chiral Plasmonics: From Static to Dynamic.Acc Chem Res. 2017 Dec 19;50(12):2906-2914. doi: 10.1021/acs.accounts.7b00389. Epub 2017 Sep 27. Acc Chem Res. 2017. PMID: 28953361
-
DNA Origami Nanomachines.Molecules. 2018 Jul 18;23(7):1766. doi: 10.3390/molecules23071766. Molecules. 2018. PMID: 30022011 Free PMC article. Review.
-
Switchable DNA-origami nanostructures that respond to their environment and their applications.Biophys Rev. 2018 Oct;10(5):1283-1293. doi: 10.1007/s12551-018-0462-z. Epub 2018 Oct 2. Biophys Rev. 2018. PMID: 30280371 Free PMC article. Review.
-
Nanomechanical molecular devices made of DNA origami.Acc Chem Res. 2014 Jun 17;47(6):1742-9. doi: 10.1021/ar400328v. Epub 2014 Apr 29. Acc Chem Res. 2014. PMID: 24772996
Cited by
-
Radiation and DNA Origami Nanotechnology: Probing Structural Integrity at the Nanoscale.Chemphyschem. 2025 Jan 2;26(1):e202400863. doi: 10.1002/cphc.202400863. Epub 2024 Nov 20. Chemphyschem. 2025. PMID: 39473163 Free PMC article. Review.
-
Realizing mechanical frustration at the nanoscale using DNA origami.Nat Commun. 2025 Jun 4;16(1):5164. doi: 10.1038/s41467-025-60492-z. Nat Commun. 2025. PMID: 40461486 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

