Rapid Detection of Pityophthorus juglandis (Blackman) (Coleoptera, Curculionidae) with the Loop-Mediated Isothermal Amplification (LAMP) Method
- PMID: 34067342
- PMCID: PMC8224600
- DOI: 10.3390/plants10061048
Rapid Detection of Pityophthorus juglandis (Blackman) (Coleoptera, Curculionidae) with the Loop-Mediated Isothermal Amplification (LAMP) Method
Abstract
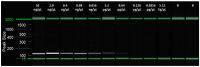
The walnut twig beetle Pityophthorus juglandis is a phloem-boring bark beetle responsible, in association with the ascomycete Geosmithia morbida, for the Thousand Cankers Disease (TCD) of walnut trees. The recent finding of TCD in Europe prompted the development of effective diagnostic protocols for the early detection of members of this insect/fungus complex. Here we report the development of a highly efficient, low-cost, and rapid method for detecting the beetle, or even just its biological traces, from environmental samples: the loop-mediated isothermal amplification (LAMP) assay. The method, designed on the 28S ribosomal RNA gene, showed high specificity and sensitivity, with no cross reactivity to other bark beetles and wood-boring insects. The test was successful even with very small amounts of the target insect's nucleic acid, with limit values of 0.64 pg/µL and 3.2 pg/µL for WTB adults and frass, respectively. A comparison of the method (both in real time and visual) with conventional PCR did not display significant differences in terms of LoD. This LAMP protocol will enable quick, low-cost, and early detection of P. juglandis in areas with new infestations and for phytosanitary inspections at vulnerable sites (e.g., seaports, airports, loading stations, storage facilities, and wood processing companies).
Keywords: bark beetle; invasive species; molecular identification; thousand canker disease; walnut.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Tisserat N., Cranshaw W., Leatherman D., Utley C., Alexander K. Black Walnut Mortality in Colorado Caused by the Walnut Twig Beetle and Thousand Cankers Disease. Plant Heal. Prog. 2009;10:10. doi: 10.1094/PHP-2009-0811-01-RS. - DOI
-
- Seybold S.J., Dallara P.L., Hishinuma S.M., Flint M.L. Detecting and Identifying the Walnut Twig Beetle: Monitoring Guidelines for the Invasive Vector of Thousand Cankers Disease of Walnut. [(accessed on 15 January 2021)];2013 Available online: http://ipm.ucanr.edu/PDF/PESTNOTES/WTB_trapping.pdf.
LinkOut - more resources
Full Text Sources

